Go To Index
Chemistry - Paper II
For Class XII (Science Group)
Model papers 2020 -2021
X11 - CHEMISTRY MODEL PAPER SOLUTION
Section "B“ (Short Answer Questions (Marks: 24)
Note: Attempt any six part questions, Three from organic and Three from inorganic chemistry. All questions carry equal marks.
INORGANIC CHEMISTRY
Q.2: (i) Refer to the list of given compounds.
Ans:
a) Formula:
Formula Of A (Dolomite) = MgCO
3 . CaCO
3
Formula Of B (Whiterite) = BaCO
3
b) Equation when C (Blue Vitriol or Copper sulphate or CuSO4) heated up to 230 °C.
{Note: Copper sulphate crystalline is blue in color which on heating loses all its water of crystallization slowly changing into colourless from (anhydrous)}
c) Chemical Formula and use of D (Potash Alum or Phitkari)
Chemical Formula of D (Potash Alum or Phitkari) = K
2SO
4.Al
2(SO
4)
3. 24H
2O
Uses Of Potash Alum:
The following are uses of potash alum:
1.
Fire Retardant:
The use of potassium alum for textiles, wood and paperless flame resistance is as fire-retardant.
2.
Tanning:
For leather tanning, potassium alum is used to extract moisture from hide and avoid rotting. Alum is not covered and can be washed out, as compared to tannic acid.
3.
Iron and Steel Dissolving:
This Aluminium solution has the property that steels are dissolved without affecting Aluminium or base metals. For machined castings of steel parts of machinery, alum solutions can be used.
Ans 2: (ii) The IUPAC names of the following:
| CHEMICAL FORMULA | IUPAC NAME |
|---|
| K3[Fe(CN)6] | Potassium Hexacyano ferrate (III) |
| [Zn(OH)4]-2 | Tetra hydroxo zincate (II) |
| [Cr(NH)3Cl3] | Tri amine tri choloro chromium (III) |
| [Ni(en)2Cl2] | Dichloro bis ethylene di amine nickel (II) |
Ans 2 (iii): Hydrogen cannot be placed in Group IA and VII A of the periodic table:DIFFERENCES / DISSIMILARITIES WITH GROUP IA
1. The physical state of IA group element is solid, where as hydrogen is a gas.
2. Hydrogen is nonmetal but the elements of group IA are all the metals.
3. The alkali metals (Group IA elements) exist in mono atomic state (Li, Na, K etc), while Hydrogen always exist in diatomic state i.e H
2.
4. Hydrogen needs only 1 electron to complete its outer most shell but all alkali metals need 7 electrons to complete their outer most shell.
5. Alkali metals forms ionic bonds but hydrogen can form both ionic and covalent bonds.
DIFFERENCES / DISSIMILARITIES WITH GROUP VIIA:
1. Hydrogen belongs to s-block elements while VII A elements belong to p-block elements.
2. Hydrogen has one electron in their outer most shell but elements of group VII A has seven electrons in their outer most shell.
3. Electron affinity of hydrogen is much less than halogens (Group VIIA elements).
4. Hydride ion (H
-) is unstable whereas halide ions (X
-1 = Cl
-1, Br
-1, I
-1) are stable one.
5. Halogen with carbon form polar covalent while Hydrogen with Carbon form non-polar molecule.
6. Element of group VIIA are oxidizing agent while hydrogen is reducing agent.
(Note: Write down any 4 differences as mentioned in question for each Hydrogen and group IA and Hydrogen and group VIIA)Ans 2 (iv): Groups of the periodic table that have following ground state electronic configuration in their outer most
shell:
| Electronic Configuration | Groups / Period / Blocks |
|---|
| 3s2, 3p2 | Group = IVA / Period = 3rd / Block = p |
| 3s2, 3p6, 4s1 | Group = IA / Period = 4th / Block = s |
| 4s2, 3d1 | Group = IIIB / Period = 4th / Block = d |
| 4s2, 3d10, 4p5 | Group = VIIA / Period = 4th / Block = p |
(Note: In question only group is asked.)
Ans 2 (v): Extraction Of Sodium From Rock Salt On Industrial Scale:
Sodium metal is commercially obtained by molten sodium chloride by Down's process.
PRINCIPLE:
The basic principle of Down's Cell is electrolysis of Molten or Fused Sodium Chloride to produce metallic sodium.
CONSTRUCTION OF DOWN'S CELLS:-
Down's Cell is made up of Iron graphite in the center which is used as anode to collect the chlorine gas (Cl2).
-
The cathode is made up of circular bar or bent bar of Cu or Fe, which surround the anode.
-
They both are separated by the iron gauze to prevent Na+ and Cl- to come in contact.
CONDITION:-
The melting point of NaCI is high that is 801°C. Calcium Chloride is added to decrease the temperature to 600°C. (Scientific Reason).
-
Electrolysis is carried out in the absence of water.
WORKING OF DOWN'S CELL:-
A mixture of NaCl and CaCl2 is introduced in the cell. As the electric current is passed through electrolyte, molten Sodium Chloride is ionized into Sodium Ion and Chloride Ion.
-
Sodium ion being positive in charge moves towards cathode while chloride ion moves towards Anode.
-
At Cathode Reduction occurs which result in storage of Sodium. The Na in molten state rises up and stored in inverted through. The 99.9% pure sodium obtained through this procedure.
CHEMICAL REACTION IN CELL:
IONIZATION:NaC1(l) ⇌ Na(l)+ + Cl(l)-
AT CATHODE: (Reduction)2Na+ +2e- ⟶ 2Na
AT ANODE: (Oxidation)2Cl- ⟶ Cl2 +2e-
ADVANTAGES OF DOWN'S CELL:
It has following advantages.
-
An expensive and important byproduct Cl gas is obtained.
-
Liquid sodium can be obtained easily at 600 °C.
EXTRACTION OR METALLURGY OF SODIUM IN DOWN'S CELL
Ans 2 (vi) Equation)
a) Nitric acid react with Phosphorous:
Nitric acid oxidizes non-metals and it is reduced to NO
2.
P + HNO3 ⟶ H3PO4 + 5NO2 + H2O
Phosphorous + Nitric acid ⟶ Phosphoric acid + Nitrogen Dioxide + Water
b) Sodium react with oxygen2Na + O2 ⟶ Na2O2 (Nitrogen per oxide)
c) Carbon monoxide is treated with chlorine
Chlorine give addition reaction with Carbon monoxide
CO + Cl ⟶ COCl2 (Carbonyl chlorid or Phosgene)
d) Aluminum is treated with Chlorine 2Al + 3Cl2 ⟶ 2AlCl3 (Aluminium chloride)
ORGANIC CHEMISTRY
Ans 2 (vii): Definitions:
1. GLYCOSIDIC LINKAGE:
A glycosidic bond or glyeasidIc linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
2. PLASTICIZER:
A plasticizer is a substance that is added to a material to make it softer and more flexible, to increase its plasticity, to decrease its viscosity or to decrease friction during its handling in manufacture.
3. AROMATICITY:
Aromaticity is a property of cyclic (ring-shaped), planar (flat) structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to other geometric or connective arrangements with the same set of atoms.
4. HOMOLOGOUS SERIES:
A series of organic compounds that have similar structural features but differ from adjacent members by (-CH
2) group is referred to as homologous series. Each member of homologous series is called homologous.
Ans 2 (viii): Definition Of The Polymerization and Isomerism.
POLYMERIZATION:
DEFINITION: A self addition reaction in which a number of simple molecules (monomers) are joined to form a very large molecule is called polymerization.
OR
A chemical reaction in which monomers are converted into polymer is called as polymerization.
ISOMERISM:
DEFINITION: Compounds having same molecular formula but different structural formula and differ from each other in physical and chemical properties are known as "Isomers" and this phenomenon is called isomerism.' Isomerism is due to the difference in the arrangement of atoms in molecules.
b) Compounds as Isomers and Polymers
| COMPOUNDS | Isomers / Polymers |
|---|
| Glucose and Starch | Polymer |
| CH3-0-CH3 and CH3-CH2-OH | Isomer (Functional Groups) |
| CH3-CH2-CHO and CH3-CO-CH3 | Isomer (Functional Groups) |
| Vinyl Chloride and PVC | Polymer |
Ans 2 (ix): Preparation Of Compounds:
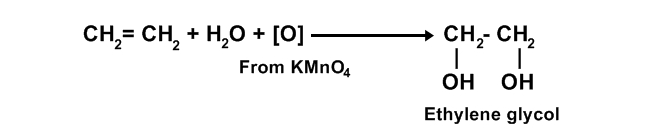
a) Ethylene glycol from ethene:
When ethene is added in dilute and alkaline solution of KMnO
4, ethene is oxidize to ethane 1-2 diol (ethylene glycol) and purple color of KMnO
4 is decolourized.
b) Phenyl hydrazone from formaldehyde:
Formaldehyde (H
2C=O) adds phenyl hydrozine in normal manner to form stable product called phenyl hydrazone (H
2C=N- NH-C
6H
5).
(i) H2C=O + H2N-NH-C6H5 ⟶ OH-CH2- NH- NH-C6H5 (unstable addition product)
(ii) OH-CH2- NH- NH-C6H5 ⟶ H2C=N- NH-C6H5 (phenyl hydrazone)
c) White solid from Acetylene:
When acetylene is heated with AgNO
3 in Ammonia Silver acetylide (White solid) is formed.
CH=CH + 2CuClaq ⟶ AgC=CAg + 2HNO3
Acetylene (Ethyne) + cuprous chloride ⟶ Silver acetylide (White solid) + Nitric acid
d) Ethane from chloro methane:
When Chloro methane is treated with sodium metal, ethane is formed.
2CH3 Cl + 2Na ⟶ C2H6 + 2NaCl
e) Ethene from ethane:
i) By pyrolysis of ethane:
When ethane is heated to high temperature (500 °C) in the absence of air, a thermal decomposition occur to give ethane and hydrogen.
CH3-CH3 ⟶ CH2=CH2 + H2
OR
ii) By halogination of ethane:
First ethane react with halogen (Cl, Br) in the presence of sunlight to produce ethyl halide than ethyl halide react with alcoholic potassium hydroxide (KOH) to give ethene.
Ans 2 (x): IUPAC names of the Componds
| Compounds | IUPAC Names |
|---|
| CH3-CH(CH3)-CH(CH3)-CH3 | 2,3 di methyl butane |
| CH2=C(CH3)-CH(CH3)=CH | 2,3 di methyl 4-yne — 1-pentene |
| CHI3 | Tri iodo methane (Iodoform) |
| CH3-CH(CH3)-CH(Cl)-CHO | 3-methyl 2-chloro butanal |
| (CH3)3-C.CO-CH2CH3 | 4.4-dimethyl pent - 3 - one |
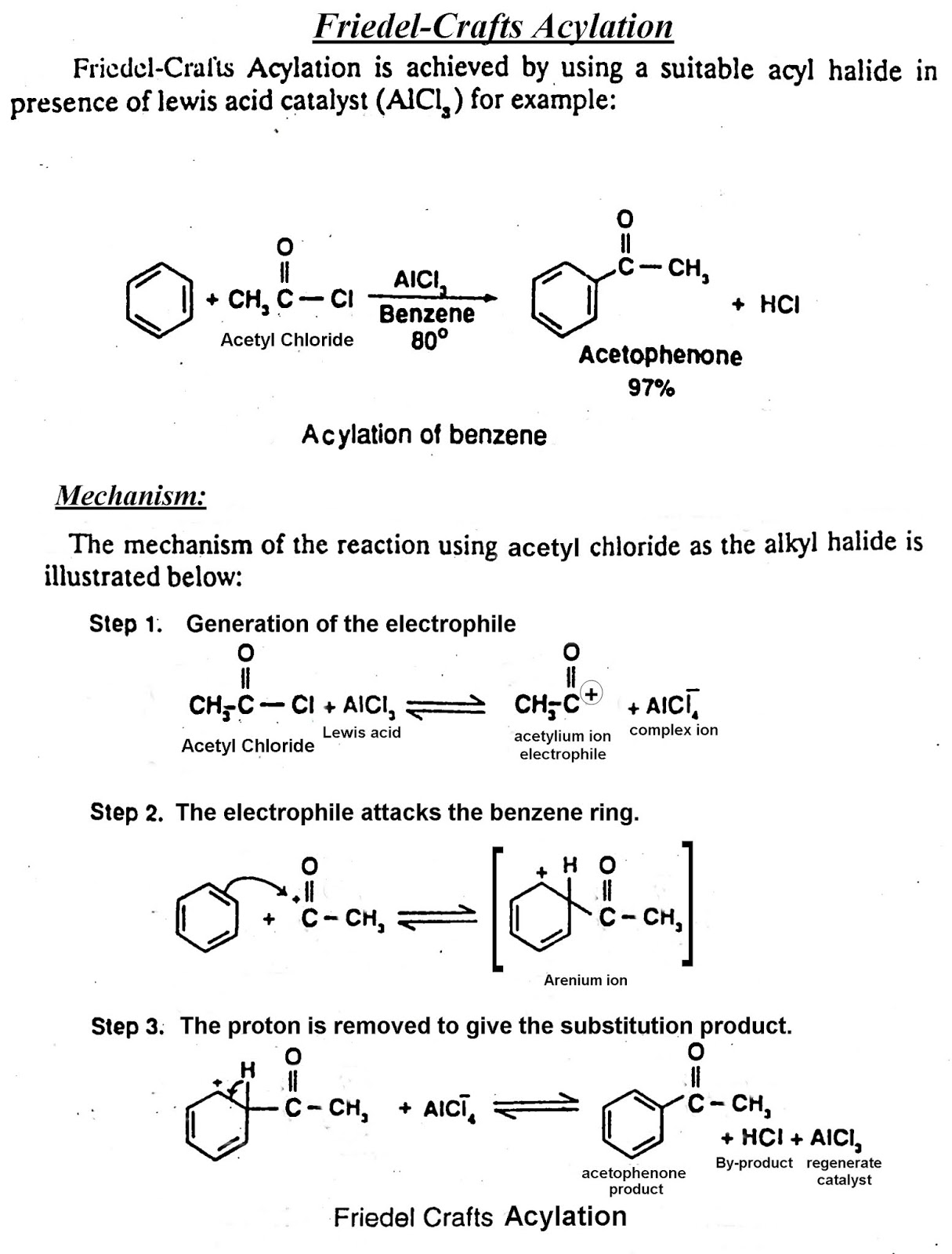
Ans 2 (xi): Benzene Gives Electrophilic Substitution Reaction:
Benzene molecule consist of three alternative double bond in a cyclic planar structure, having three pairs of delocalized electrons above and below the plane of ring. Hence, it is electron-rich. The resonance energy of benzene is very high 36 Kcal per molecule. As a result, it is highly attractive to electron deficient species i.e. electrophiles. Therefore, it undergoes electrophIlic substitution reactions very easily.
Acylation Of Benzene By Friedel-Craft Reaction:
The introduction of The acyl group (R-C=O), in the benzene ring is generally achieved with the help of Lewis acid catalyst as AlCl
3. This reaction is known as Friedel-Craft Reaction.
Ans 2 (xii): Equations:
a) Acetylene reacts with water in the presence of H2SO4 and HgSO4 at 75 °C.
b) Formaldehyde is polymerized in presence of H2SO4.
c) Vapors of acetic acid are passed over MnO2 at 500 °C.
d) Ethanol in excess, is heated in presence of H2SO4.
OR
Short Note On Amino Acid Or Fertilizers
FERTILIZERS:
"Fertilizers are commonly inorganic salts and containing elements such as nitrogen, phosphorus, potassium etc, which are very essential for the growth and development of plants. The yield of agricultural crops can be increased by introducing fertilizers to the soil."
NEED OR USES OF FERTILIZERS:
Fertilizers stimulate the process of metabolism is the plant cells. The need of fertilizers are due to three reasons.
-
To make up the deficiency of elements (like N, P, K) and become fertile again.
-
To give an additional supply of food.
-
To maintain pH of the soil near neutrality or slightly alkalinity i.e from pH 7 to 8. The soil having pH above 10 or below 3 is sterile.
CLASSIFICATION OF FERTILIZERS:
There are two types of fertilizers, depending upon the source and chemical nature:
-
Natural or Organic Fertilizers:
These are derived from Plant and animals and may be organic or inorganic. They includes manures, peats, hay wastage and rock phosphate and Chillie salt peter etc
Uses: Natural fertilizers provide more nutrient than synthetic fertilizers.
-
Synthetic or Mineral Fertilizers:
Mineral fertilizers are obtained from mineral raw materials. These are preferred on natural fertilizers because they contain exact percentage of elements (e.g. Nitrogen and Phosphorus) as required for an specific crop. They are also called artificial fertilizers.
The Mineral fertilizers are categorized as:
a) Nitrogen Fertilizers:
Fertilizers containing nitrogen as essential elements are called "Nitrogenous Fertilizers".
- Urea (NH2 - CO - NH2)
- Ammonia NH3
- Ammonium Nitrates (NH4NO3)
- Ammonium Sulphate {(NH4)2SO4}
Importance or Uses of Nitrogen:- It is necessary in the early stage for the rapid growth of plants.
- It is main constituent of proteins.
b) Potassic Fertilizers:
Fertilizers containing potassium as essential element are called "Potassic Fertilizers", such as:
- Chile saltpeter or Potassium Nitrate, KNO3.
- Potassium Sulphate K2SO4.
Importance or Uses of Potassium:- It is required in the formation of starch, sugar and fibrous material.
- It resist plant diseases.
- It makes plant strong by making root healthy.
- It helps in ripening of seeds, fruits and cereals.
c) Phosphatic Fertilizers:
Fertilizers containing phosphorous as essential element are called "Phosphatic Fertilizers."
The important phosphorus fertilizers are given below:
- Amonium phophate:
e.g: (NH4)2PO4 - Diamonium hydrogen phophate:
e.g: (NH4)2HPO4 - Super Phosphates:
e.g: Mixture of Ca(H2.PO4)2 and CaSO4
- Triple phosphate:
e.g: 3Ca(H2.PO4)2
Importance or Uses of Phosphorus:- It stimulates the early growth and development of plants.
- It accelerates the seed and fruit formation processes.
- It also resist plant against diseases and frost.
SCOPE OF FERTILIZERS IN PAKISTAN:
Pakistan is an agricultural country It does not only prepare fertilizers of its own demand but also exports in a bulk.
INDUSTRIES IN PAKISTAN:
Some important fertilizer factories of Pakistan are enlisted below:-
- TSF Plant and urea fertilizer plant, Hazara.
- Faisalabad Fertilizer Ltd.
- Pak American Fertilizer Ltd. at Daud Khel.
- Single Super Phosphate Plant at Jaran Wala.
- Natural Gas Fertilizer Factory, Multan.
- Dawood Urea Plant, Lahore.
- Dhariala Potash Fertilizer Project, Dhariala.
- Fauji Urea Complex, Sadiqabad.
- Exxon Fertilizer Co. Dahrki.
- Urea Plant, Mirpur Mathelo.
(Note: write down only any two industries in notes to save time for others question in exams)
AMINO ACID:
DEFINITION:
Amino acids are bi-functional compounds containing both carboxylic acid group (-COOH) and basic amino group (-NH
2). The general formula of amino acid is R-CH(NH
2)-COOH.
They are building blocks of proteins. They arc linked together by peptide bond
TYPES OF AMINO ACID:
There are two types of amino acids:
1. Essential Amino Acids:
Those which cannot be synthesized by human body but they are essential for:
i) growth of infants
ii) Transmission of impulses in the nervous system
are called "Essential Amino acids".
Example: Leucine, Isoleucine, Methionine etc.
These amino acids must be supplied to our body through diet and their deficiency cause diseases.
2. Non-Essential Amino Acids:
Those which can be synthesized by human body are called "Non-essential Amino Acids". There are 20 amino acids required for synthesis of protein. Out of which 10 are essential and 10 are non essential amino acids.
CLASSIFICATION OF AMINO ACIDS:
Amino acids are classified according to their nature i,e. neutral, acidic or basic.
1. NEUTRAL AMINO ACIDS:Amino acids containing one amino group and one carboxyl group are called "Neutral Amino Acids". Amino group acts as basic and carboxyl as acidic, both neutralize each other. Hence amino acids exhibit neutral characteristics and amphoteric nature.
For example:
i. Glycine NH
2-CH
2-COOH
ii. Alanine CH
3-CH(NH
2)-COOH
iii. Valine CH
3-CH(CH
3)-CH(NH
2)-COOH
2. ACIDIC AMINO ACIDS:
Amino acids containing one amino group and more than one carboxyl group are called "Acidic Amino Acids". Acidic amino acids exhibit acidic nature.
For example:
i. Aspartic Acid HOOC-CH(NH
2)-CH
2-COOH
ii. Glutamic Acid HOOC-CH(NH
2)-CH
2-CH
2COOH
3. BASIC AMINO ACIDS:
Amino acids containing one hydroxyl group and more than one amino group are called "Basic Amino Acids". Basic amino acids exhibit basic nature.
For example:
i. Arginine HOOC-CH(NH
2)-(CH
2)
3-NH-C(NH)-NH
2
ii. Lysine HOOC-CH(NH
2)-(CH
2)
3-NH
2
ZWITTER (Dipolar Nature of Amino Acids):
A dipolar charged but electrically neutral ion is called Zwitter Ion". In amino acids. carboxyl (acidic) group ionizes to donate proton H
+ whereas amino (basic) group having lone pair of electron behaves as proton acceptor becoming Lewis Base. Hence amino acids exists as dipolar ion in an un-ionized form called "Zwitter Ion".
Due to Zwitter Ion, amino acids have following properties:
1) Amino acids are soluble in water but insoluble in organic solvents.
2) Amino acids are solids.
3) Amino acids have high melting point and donate or accept proton H
+.
PEPTIDE BOND:
Amino acids in proteins are linked together through an acid-amide group type of bond known peptide bond. The peptide bond is formed between two amino acid molecules when amino group of one amino acid is linked with the cathoxylic group to other amino acid molecule by the elimination of water molecule.
ROLE OF AMINO ACID IN HUMAN BODY:
When food containing protein is taken, many enzymes act on protein and completely hydrolyse it into amino acids. These amino acids are absorbed in blood which carries them to cells where any one of following action take place:
1) amino acids can be synthesized back to body protein.
2) oxidation may take place to provide energy.
3) body protein may be transformed into carbohydrates or fats in case of low amount in diet or make harmones and other body necessities.
SOURCE: Board Of Intermediate Education Karachi