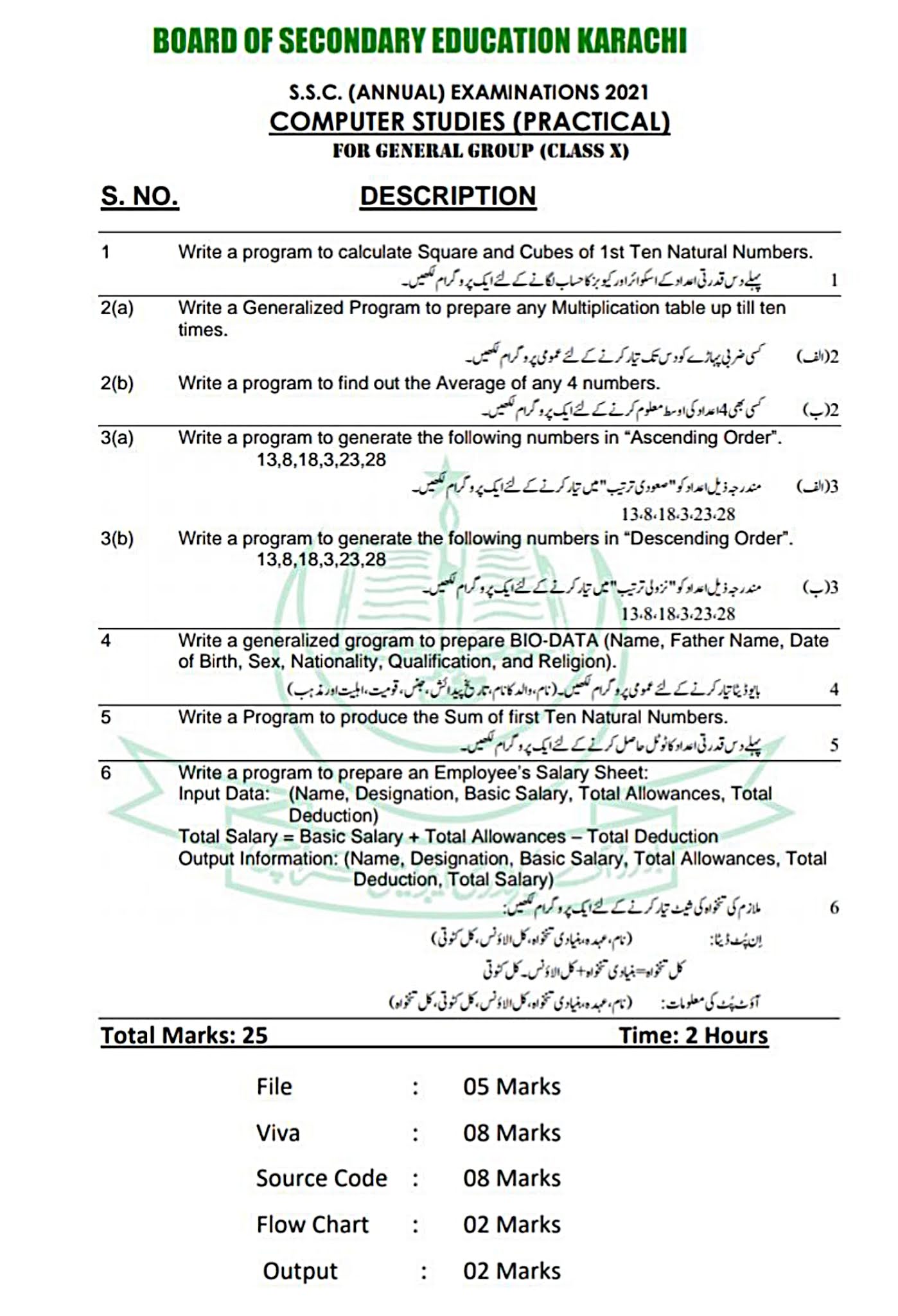
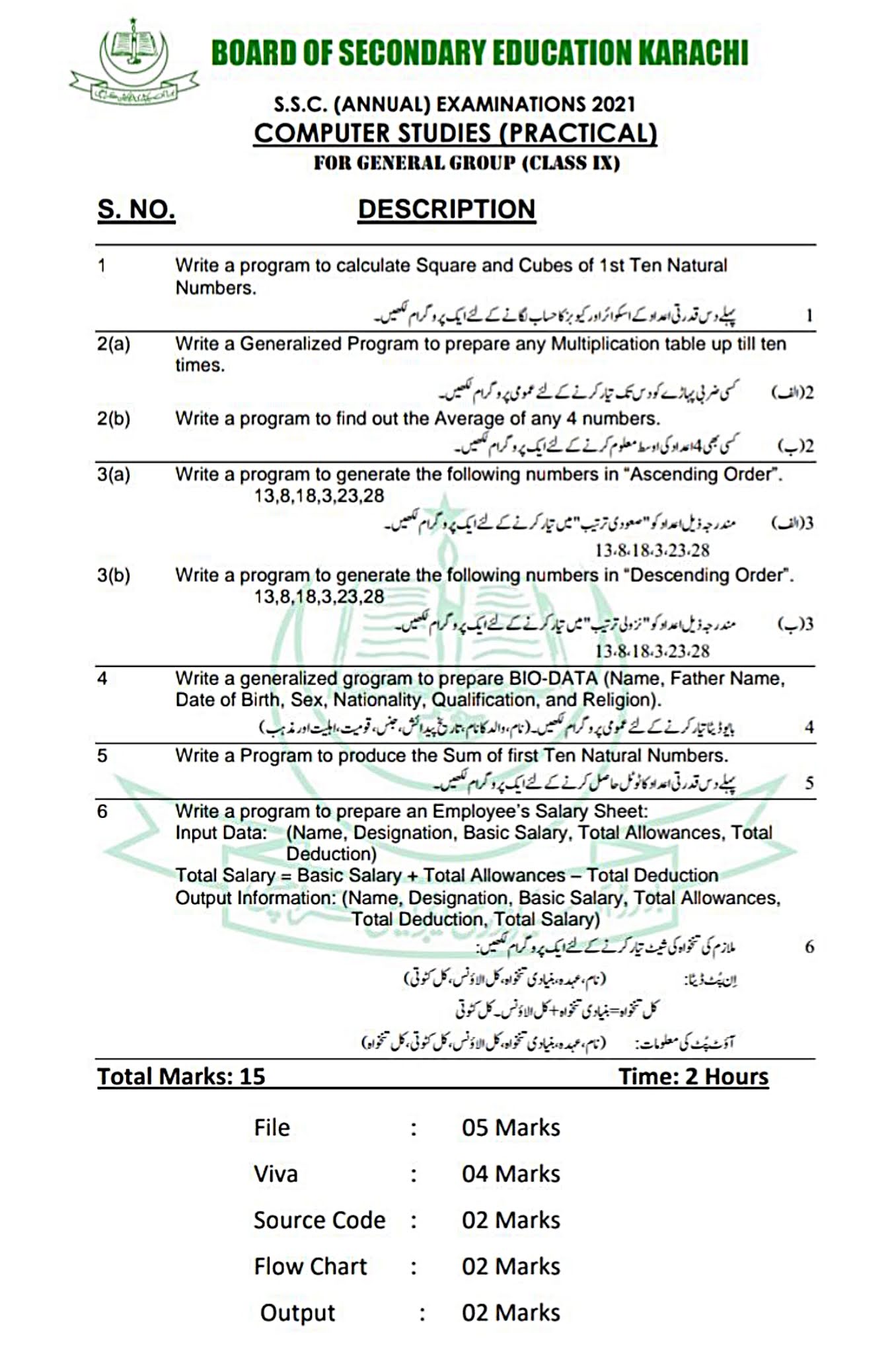
Go To Index
EXPERIMENT NO.5
TO PREPARE THE STANDARD SOLUTION OF OXALIC ACID
APPARATUS:
- Measuring flask
- Filter paper
- Funnel
- Beaker
- Glass rod
- Pipette
- Physical balance
- Weight box
CHEMICALS:
- Oxalic acid (COOH)2.2H2O
- Distilled water
THEORY:
CONCENTRATION:
The amount of solute present in definite volume of solution is called concentration of a solution.
MOLARITY:
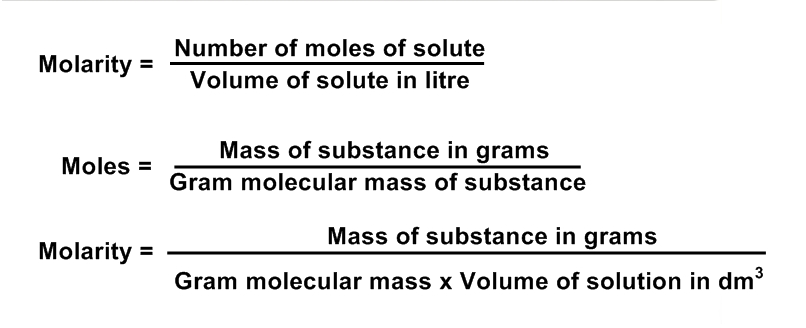
It is defined as, the number of moles of solute dissolved per litter or per cubic decimetre of solution. lt is denoted by "M".
FORMULA OF MOLARITY:
FORMULA OF MASS:
1. Mass of substance = Molarity x gram mol mass x volume in dm3
2. Mass of substance = Molarity x gram molecular mass x volume in cm3 / 1000
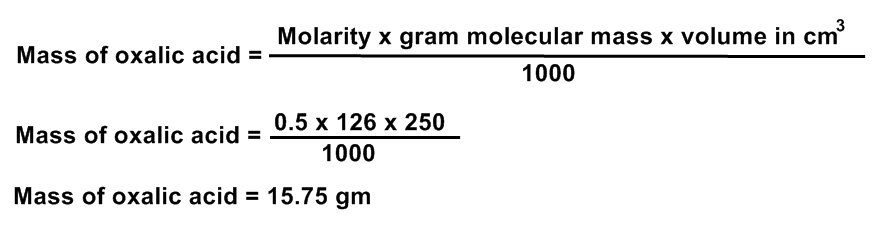
CALCULATIONS FOR 0.5 M SOLUTION OF OXALIC ACID
1. MOLECULAR FORMULA OF OXALIC ACID
(COOH)2.2H2O
2. CALCULATION OF MOLECULAR MASS OF OXALIC ACID
(COOH)2.2H2O
= (12 + 16 + 16 + 1)2 + 2 (2 + 16)
= ( 24 + 32 + 32 + 2) + 2(18)
= 90 + 36
= 126 gm
3. MASS OF OXALIC ACID REQUIRED FOR 100 ML
4. MASS OF OXALIC ACID REQUIRED FOR 250 ML
METHOD:
- Adjust physical balance.
- Place filter paper in left hand pan.
- With the help of forceps place standard weights of weight box in right hand pan, till the pointer oscillates equally about centre zero, when the centre rod is raised.
- Record mass of filter paper.
- Now place the weighed filter paper in right hand side pan and weights in left hand side pan.
- Place additional weights (6.3 gm for 100 ml or 15.75 gm for 250 ml in left hand pan.
- Put oxalic acid in right hand pan equivalent to the weights (6.3 gm or 15.75 gm).
- Transfer weighed oxalic acid into a beaker and dissolve it in a minimum quantity distilled water by stirring with a glass rod.
- Take a 100 ml flask for 6.3 gm or 250 ml flask for 15.75 gm oxalic acid.
- Transfer the solution from beaker into the flask.
- Wash the beaker and transfer the weighing to the flask.
- Add more water into the flask till the level of solution reaches very close to mark on the neck.
- Now continuously add water with the help of pipette, dropper or wash bottle till the lower meniscus touches the required mark.
- This is 0.5 M solution of oxalic acid.
PRECAUTIONS:
- Physical balance must be adjusted.
- Air bubbles must be removed.
- The weighing should be accurate.
- The beaker should be washed and washing should be added to the solution of flask.
- Final addition of water should be cautiously done.
- Lower meniscus A solution should be read.
Special Thanks to Sir Sajjad Akber Chandio