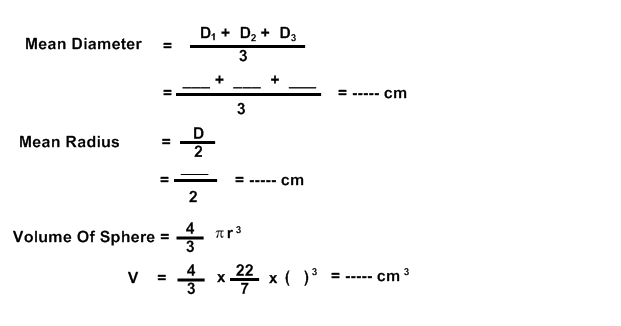Search This Blog
Saturday 31 July 2021
Chapter No.11 : Ecosystems - Botany - For HSC Part 2 (XII) - Science Group
GO TO INDEX
Chapter No.11
ECOSYSTEMBotanyFor HSC Part 2 (XII) Science Group
Q.4: Explain the climatic factors of an ecosystem.ECOSYSTEM
Ans: CLIMATIC FACTORS OF AN ECOSYSTEM:
It consists of::
- LIGHT
- TEMPERATURE
- WATER
- ATMOSPHERE AND WIND
- FIRE
1. LIGHT:
- Light is an important factor of life.
- The main source of light ss the sun.
- Light is essential for green plants for photosynthesis.
- Only 3% of total sunlight, which fulls on earth, is utilized.
- The intensity, duration and quality of light is important for living organisms.
i) LIGHT INTENSITY:
- Light intensity varies with seasons by light intensity.
- Opening and closing of stomata and permeability of cell membrane is affected by light intensity.
- Intense light destroys chlorophyll.
- The plants growing In light arc called helophytes while those growing in shade are called sclophytes.
ii) LIGHT DURATION:
- The light duration or photoperiod has marked-effect on flowering, leaf fall and dormancy in plants.
- In animals, behavioral activities (biological rhythms) such as court ship display, nesting and other behaviours are also effected by light.
- In spring, migration of certain groups of birds depend upon photoperiod.
iii) LIGHT QUANTITY:
- The visible light which ranges from 400-780 mn, consists of seven colours. The chlorophyll absorbs red and blue green colour for photosynthesis.
- Light with shorter wavelength carries more energy but destroys micro-organisms and also causes sunburn in human beings.
2. TEMPERATURE:
- A moderate temperature favours the growth and other activities of plants and animals.
- Life exists, from 0°C to 50°C.
- Different animals found at different places are due to variation in temperature.
- Temperature below freezing. point or high temperature kills the living organisms or damage them.
- Optimum temperature is required for enzymes efficiency.
3. WATER:
- Water is the most important factor of ecosystem and is an important need of all the living organisms.
- According to the availability of water, plants are grouped into XEROPHYTES, HYDROPHYTES and MESOPHYTES.
- Water is abundant in aquatic ecosystem while it becomes limited in terrestrial ecosystem.
- Water Is needed for all the physiological activities of plants and animals.
- It is one of the raw materials needed for photosynthesis.
- In terrestrial ecosystem, rainfall is the main source of water.
- Plants absorb it from soil, however its availability is related to many factor like edaphic factors of soil and kind of vegetation.
- In nature continuous cycling of water also takes place, it is culled hydrological cycle.
4. ATMOSPHERE AND WIND:
- Atmosphere is u store house of CO2, O2, N2 and vapours of water.
- O2 is used In respiration.
- CO2 in used in photosynthesis and N2 is a major component of protein.
- Wind current distributes these gases and brings atmospheric circulation of water vapours.
- Wind also favour pollination and dispersal of seeds.
- Wind with high velocity breaks the branches and plants.
- Wind also influences the migration of flying birds and restricts timberline.
- At high altitude the air pressure and percentage of oxygen is low that's why in these areas mammals cannot live.
5. FIRE:
- Fire is n physical factor, which brings a sudden change in ecosystem.
- A Fire may be caused by lightening, volcanic activities rind mutual friction between trees such as bamboos and mostly by man.
- A forest which is established and created in centuries is destroyed in few minutes by fire.
- Fire favour the competition among trees and set new species as climax.
- Fire recycles various nutrients and new growth is stimulated.
- Fire also favours the growth of some fungi. These fungi are called Pyrophilous Fungi.
- Pyrophilous fungi decompose unborn dead plants or animals.
- Men made fires are set to clear ground for agriculture or making mud but this is the destruction of ecosystem.
Saturday 24 July 2021
Physics Practicals - For Class IX and X (Science Group) - PRACTICAL 02: Determine the Resultant of two vectors by (Gravesend's apparatus).
Go To Index
PRACTICAL 02:
- Determine the Resultant of two vectors by (Gravesend's apparatus).
APPARATUS:
-
Gravesend's Apparatus i.e:
i) Vertical drawing board
ii) Two fixed pulleys (clamped to the top) - Three sets of 20 gm slotted weights with hanger
- Plane mirror strip
- Drawing paper
- Thumb pin
- Light string about 1 meter long
- Meter Scale
- Geometry box
THEORY:
Those physical quantities, which are completely, specified only when direction is also mentioned along with magnitude and units are known as vector.
RESULTANT VECTOR:
Vectors, which gives combined effect of two or more vectors, called resultant vectors.
TRIANGULAR LAW OF VECTOR ADDITION:
If two vectors represent two sides of the triangle, then third side of triangle in reverse order will be the resultant of the two sides.
PARALLELOGRAM LAW OF VICTOR ADDITIONS:
According to this law the diagonal of parallelogram represents the resultant of two adjacent of the parallelogram.
METHOD: (1 + 1 MARKS)
- Fix drawing paper in the middle of the vector board and place it vertically.
- Adjust the thread, carrying a set of weights at each end, over two pulleys.
- The small thread carrying third set of weights at the center of the first two thread.
- Place plane mirror under one of the thread keeping one eye close and mark two points 'A' and 'B', such that the two points and the image of thread lie exactly under the thread.
- Similarly mark C, D and E, F under other two thread.
- Join A and B, C and D. and E and F and extend them to meet at point O.
- Select suitable scale (10 gm-wt = 1 cm) and draw arcs OP and OQ corresponding to their weights.
- Draw PX and QX parallel to OP and OQ.
- Join P and X and 0 and X.
- OX is the resultant of OP and PX.
- Find magnitude of OX by multiplying its length with the scale.
- Repeat above procedure by using unequal weights and find their resultant.
OBSERVATIONS: (1 + 1 MARKS)
| OBS | WEIGHT OF RIGHT HAND SIDE (OP) gm-wt | WEIGHT OF LEFT HAND SIDE (OQ) gm-wt | RESULTANT WEIGHT (OX) gm-wt |
|---|---|---|---|
| 1. | |||
| 2. |
FIGURE:
RESULT:
1. The magnitude of the resultant of two equal vectors each of ______ is _______ gm-wt.
2. The magnitude of two unequal vectors of _______ gm-wt and _______ is ______ gm-wt.
PRECAUTIONS:
- Board must be exactly vertical.
- Standard weights must be used.
- Pulleys must be friction less.
Special Thanks to Sir Sajjad Akber Chandio
Physics Practicals - For Class IX (Science Group) - PRACTICAL 01: Determine the Diameter of a small sphere using a Micrometer Screw Gauge and calculate its volume.
Go To Index
PRACTICAL 01:
Determine the Diameter of a small sphere using a Micrometer Screw Gauge and calculate its volume.
APPARATUS:
- Screw gauge
- Small sphere
THEORY:
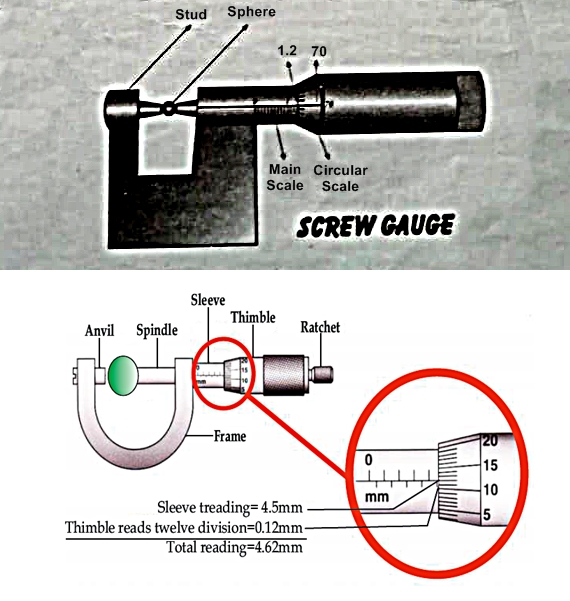
Micrometer screw gauge consists of a U - shaped steel frame of a fixed stud and a movable stud, which can move in a hollow cylinder called drum or thimble. It has two scales one is called main scale and other is called circular scale.
LEAST COUNT:
Smallest distance that can be measured by it called least count.
PITCH:
It is the perpendicular distance between the two adjacent threads.
ZERO ERROR:
When two studs of screw gauge are in contact and zero of main scale does not coincide with the zero of the circular scale then screw gauge is said to have zero error.
FORMULA: (1 MARK)
Volume of sphere = 4/3 πr3
METHOD: (2 MARKS)
- Find pitch of screw gauge by giving one rotation to circular scale.
- Calculate least count of screw gauge.
- Remove zero error if present.
- Place the sphere between studs and screw up till thc sphere heId gently. Note main scale reading and circular scale reading at line of reference.
- Multiply circular scale reading and least count to get fractional part.
- Add main scale reading and fractional part to get total diameter.
- Take three readings each time fixing the sphere from a different side.
OBSERVATIONS: (2 MARKS)
Pitch of screw gauge = 1 mm = I/10 cm = 0.1 cm
Least count = Pitch of the screw gauge / No. of circular scale divisions
L.C. = 0.1 / 100 = 0.001 cm
DIAMETER OF SPHERE:
| OBS | MAIN SCALE READING (M.S.R) cm | CIRCULAR SCALE READING (C.S.R) div | FRACTIONAL PART F.P. = C.S.R x L.C cm | TOTAL DIAMETER D = M.S.R + F.P cm |
|---|---|---|---|---|
| 1.2 cm | 70 div | 70 x 0.001 = 0.07 cm | 1.2 + 0.07 = 1.27 cm | |
| 1 | ||||
| 2 | ||||
| 3 |
CALCULATIONS: (1+4 MARKS)
RESULT:
Mean Diameter of sphere = _____ cm and
Volume of sphere = _____ cm3
FIGURE: (2 MARKS)
PRECAUTIONS:
- If there is zero error then it must be removed.
- Reading must be taken at different points of the spheres.
Special Thanks to Sir Sajjad Akber Chandio
Friday 23 July 2021
Chemistry Practicals For Class IX (Science Group) - Experiment No. 7: To test the passage of an electric current through electrolytic and non-electrolytic solutions.
Go To Index
EXPERIMENT NO.7
TO TEST THE PASSAGE OF AN ELECTRIC CURRENT THROUGH 7 ELECTROLYTIC AND NON.ELECTROLYTIC SOLUTIONS.
APPARATUS:
- Two copper strips
- Electrolytic pot or beaker
- Connecting wire
- Bulb holder
- Ammeter
- Dry cell or a battery
- Hanger
- Torch bulb
- Key
CHEMICALS:
- Copper sulphate solution
- Sodium hydroxide solution
- Benzene
- Sodium chloride solution
- Sugar solution
THEORY:
ELECTROLYTES:
Those liquids which conduct electricity are called electrolytes .e.g. solutions of copper sulphate, sodium chloride, sodium hydroxide etc.
NON- ELECTROLYTES:
Those liquids which do not conduct electricity are called non electrolytes .e.g. benzene, sugar solution etc.
METHOD:
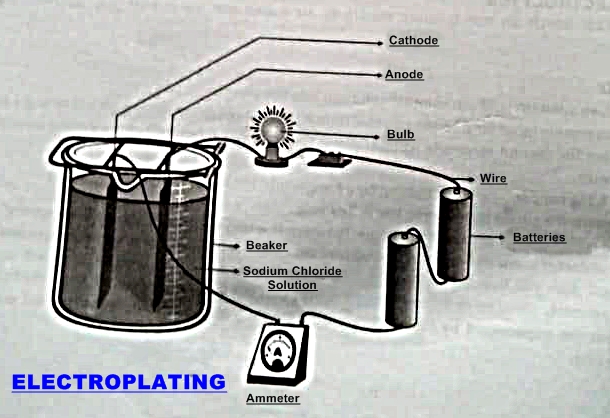
- Put given solution (sodium chloride solution) into the electrolytic pot.
- Connect one copper strip to the positive terminal of the cell (battery) through a bulb and a key.
- Connect the second electrode (copper plate) to the negative terminal of the battery through an ammeter.
- Now hang copper strips (electrodes) by a hanger into the pot of given solution.
- Complete the circuit by inserting key in key hole.
- If the current flows across the solution between the two electrodes, the bulb will start glowing and ammeter show deflection. This indicate that the given substance is an electrolyte and if the current does not flow across the solution between the two electrodes, the bulb will not glow and ammeter show no deflection. This indicates that the given substance is a non - electrolyte.
- Repeat the experiment by taking different solutions. Wash electrodes with distilled water for each test.
| S.No. | Name Of Solution | Condition Of Bulb Glowing / Not Glowing |
Condition Of Ammeter Deflecting / Not Deflecting | Nature Of Solution Electrolyte / Non electrolyte |
|---|---|---|---|---|
| 1 | Sodium chloride solution | Glowing | Deflecting | Electrolyte |
| 2 | Caustic soda solution | Glowing | Deflecting | Electrolyte |
| 3 | Sugar solution | Not Glowing | Not Deflecting | Non-electrolyte |
| 4 | Ethyl alcohol | Not Glowing | Not Deflecting | Non-electrolyte |
RESULT:
1. Sodium chloride and Caustic soda solutions are electrolytes.
2. Sugar and Ethyl alcohol solutions are non-electrolytes.
REASON:
Glowing of bulb and deflection of ammeter in case of Sodium chloride and Caustic soda solutions indicate that these solutions conduct electricity, hence these solutions are electrolyte. The bulb will not glow and ammeter will not deflect in case of Sugar and Ethyl alcohol solution. This Indicate that these solutions are non-electrolytes.
PRECAUTIONS:
- Connections should be tight.
- Electrodes should be separated from each other in electrolytic pot.
- Dilute solutions should be used.
- The electrodes should be washed with distilled water after each test.
Special Thanks to Sir Sajjad Akber Chandio
Chemistry Practicals For Class IX (Science Group) - Experiment No. 6: To standardize the given solution of sodium Hydroxide using Oxalic acid solution.
Go To Index
EXPERIMENT NO. 6
TO STANDARDIZE THE GIVEN SOLUTION OF SODIUM HYDROXIDE USING OXALIC ACID
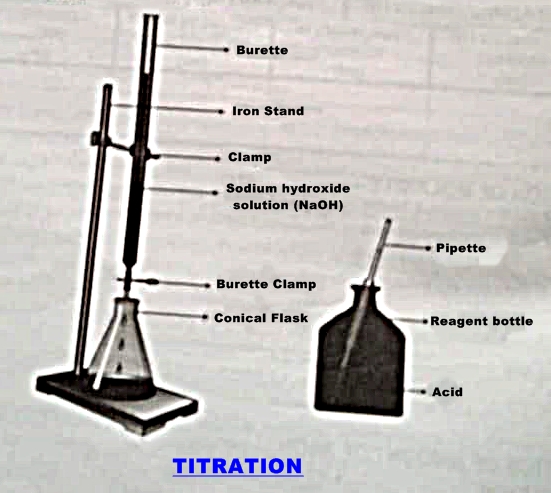
APPARATUS:
- Burette
- Beaker
- Indicator bottle
- Pipette
- Burette stand
- Conical flask
- Funnel
CHEMICALS:
- Sodium hydroxide solution
- Phenolphthalein Indicator
- Oxalic acid solution of known molarity (0.1M)
THEORY:
Titration is the method of quantitative analysis used for determining the concentration of a solution of unknown concentration using a standard solution.
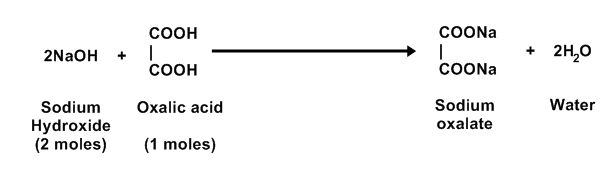
EQUATION:
METHOD:
- Wash all the apparatuses thoroughly with water. Rinse burette with a little quantity of sodium hydroxide solution.
- Rinse the pipette with oxalic acid solution.
- Clamp the burette in an iron stand vertically.
- With the help of funnel, fill the burette with sodium hydroxide solution.
- Remove the air bubbles by drawing some solution from the burette.
- Fill the burette again and assuring that the lower meniscus coincide the zero mark.
- Pipette out 10 ml of oxalic acid solution in a conical flask.
- Start the addition of alkali (NaOH) solution from the burette into the conical flask drop by drop with constant shaking until very faint pink colour persists. This is the end point.
- Record the final reading of the lower meniscus in the burette.
FIND OUT THE DIFFERENCE BETWEEN INITIAL AND FINAL READING
- Find volume of NaOH by subtracting initial reading from final reading to neutralize.
- 10 ml of oxalic acid.
- Throw the contents of conical flask and wash with water.
- Take final reading of first step as initial reading of next step.
- Repeat the procedure of titration in the same manner till three similar readings (Concordant obtained)
OBSERVATIONS:
Solution in the burette (NaOH)
Molarity of NaOH solution M1 = ?
Volume of NaOH solution (Concordant volume) V1 = ?
Number of Moles od NaOH (from equation) n1 = ?
SoIution in the conical flask (Oxalic acid)
Molarity of Oxalic acid M2 = 0.1 M
Volume of oxalic acid used for each burette reading V2 = 10 ml
No. of Moles of oxalic acid (from balanced equation) n2 = 1
Indicator used = Phenophthalein
Colour change • Colourless to light pink
Amount of NaOH in grams /litre = ?
BURETTE READING
| S.No. | INITIAL BURETTE READING (mI) | FINAL BURETTE READING (mI) | VOLUME OF NaOH SOLUTION USED (mI) | CONCORDANT VOLUME (mI) |
|---|---|---|---|---|
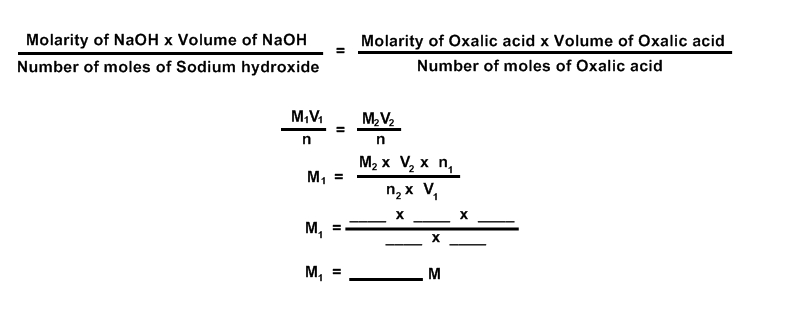
CALCULATION OF MOLARITY:
CALCULATION OF AMOUNT OF NaOH / Liter
Molar mass of NaOH = 40 g
FORMULA:
Amount of NaOH / Liter = Molarity of NaOH x Molar mass of NaOH
= M1 = 40
= ________
RESULT:
The molarity of given NaOH solution M1 = _______ M
Amount of NaOH / Liter = __________ g
PRECAUTIONS:
- Note the lower meniscus of alkali solution in the burette.
- Remove air bubble from the jet of the burette.
- Rinse the burette and The pipette with NaOH and oxalic acid respectively.
- All the should be thoroughly washed.
- Only 1 or 2 drops of indicator should be used.
- The pipette should not be down to dropout the solution.
- The lower meniscus of the solution should be read.
- The pipette should not be blown to dropout the solution.
- The flask should be shaken after each addition of the solution.
Special Thanks to Sir Sajjad Akber Chandio
Subscribe to:
Posts (Atom)