Search This Blog
Friday 10 September 2021
Physics (ENG and UR) -Paper I - Past Paper 2021 (MCQs Only) - For HSC Part 1 (Home Economics Group)
GO TO INDEX
Physics (Eng and Ur) - Paper I
Past Paper 2021 (MCQs Only)
For HSC Part 1
(Home Economics Group)
Meal Management And Food Preservation (Urdu) -Paper II - Past Paper 2021 (MCQs Only) - For HSC Part 2 (Home Economics Group) - For Failure, Improvements, Additional Subjects...
GO TO INDEX
Meal Management And
Food Preservation (Urdu)
Past Paper 2021 (MCQs Only)
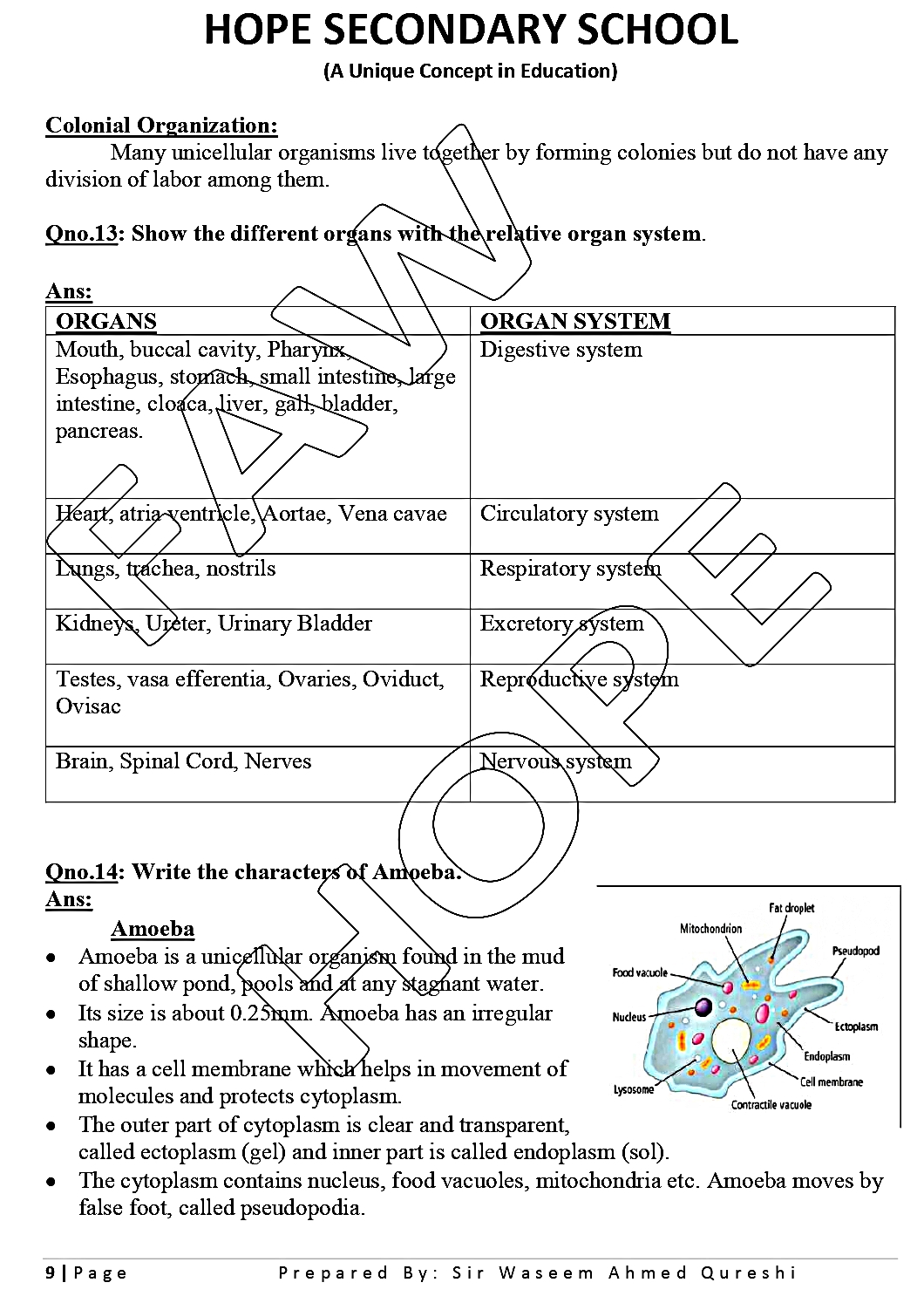
For HSC Part 2 - Home Economics Group
For Failure, Improvements, Additional Subjects...
Meal Management And Food Preservation (ENGLISH) -Paper II - Past Paper 2021 (MCQs only) - For HSC Part 2 (Home Economics Group) - For Failure, Improvements, Additional Subjects...
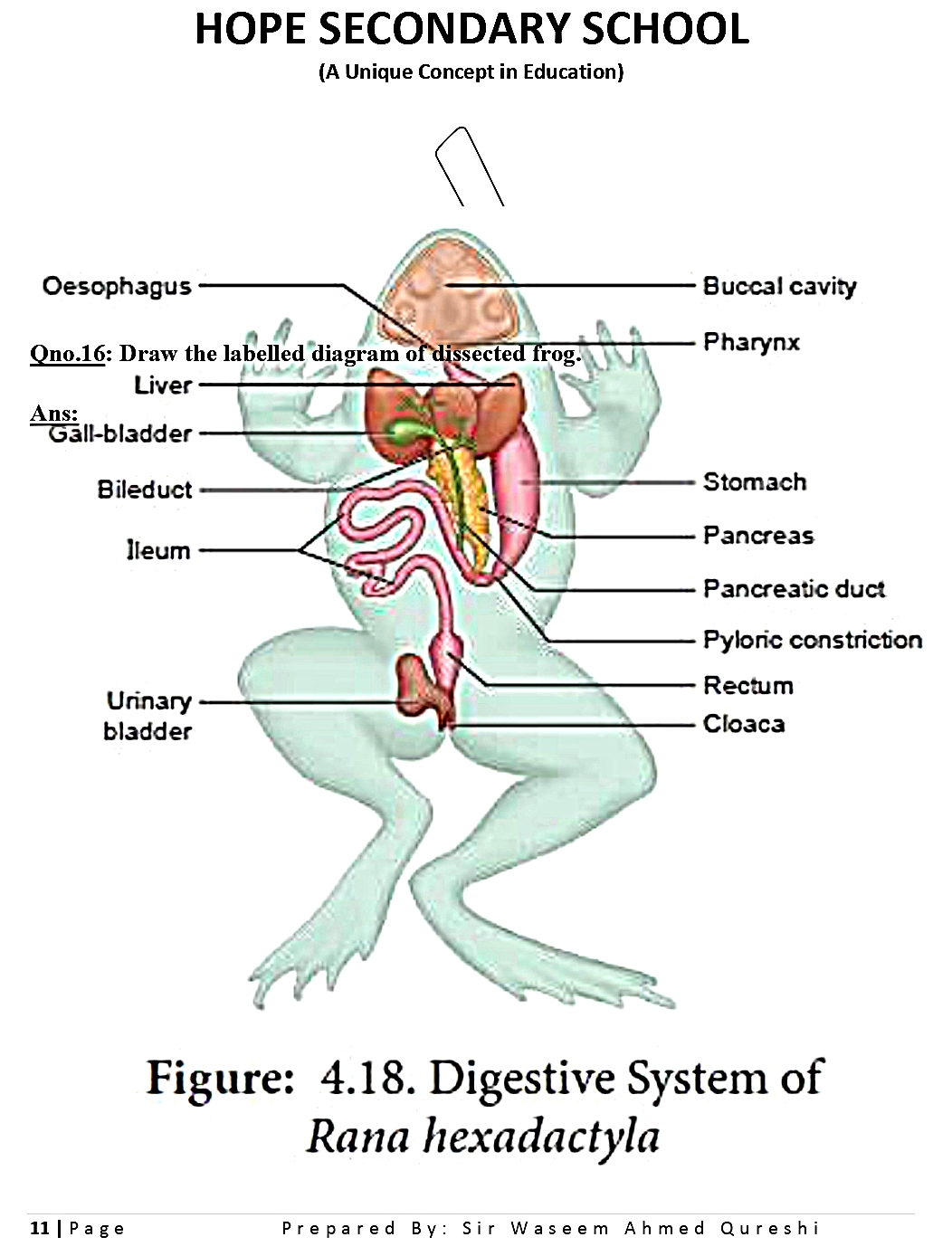
GO TO INDEX
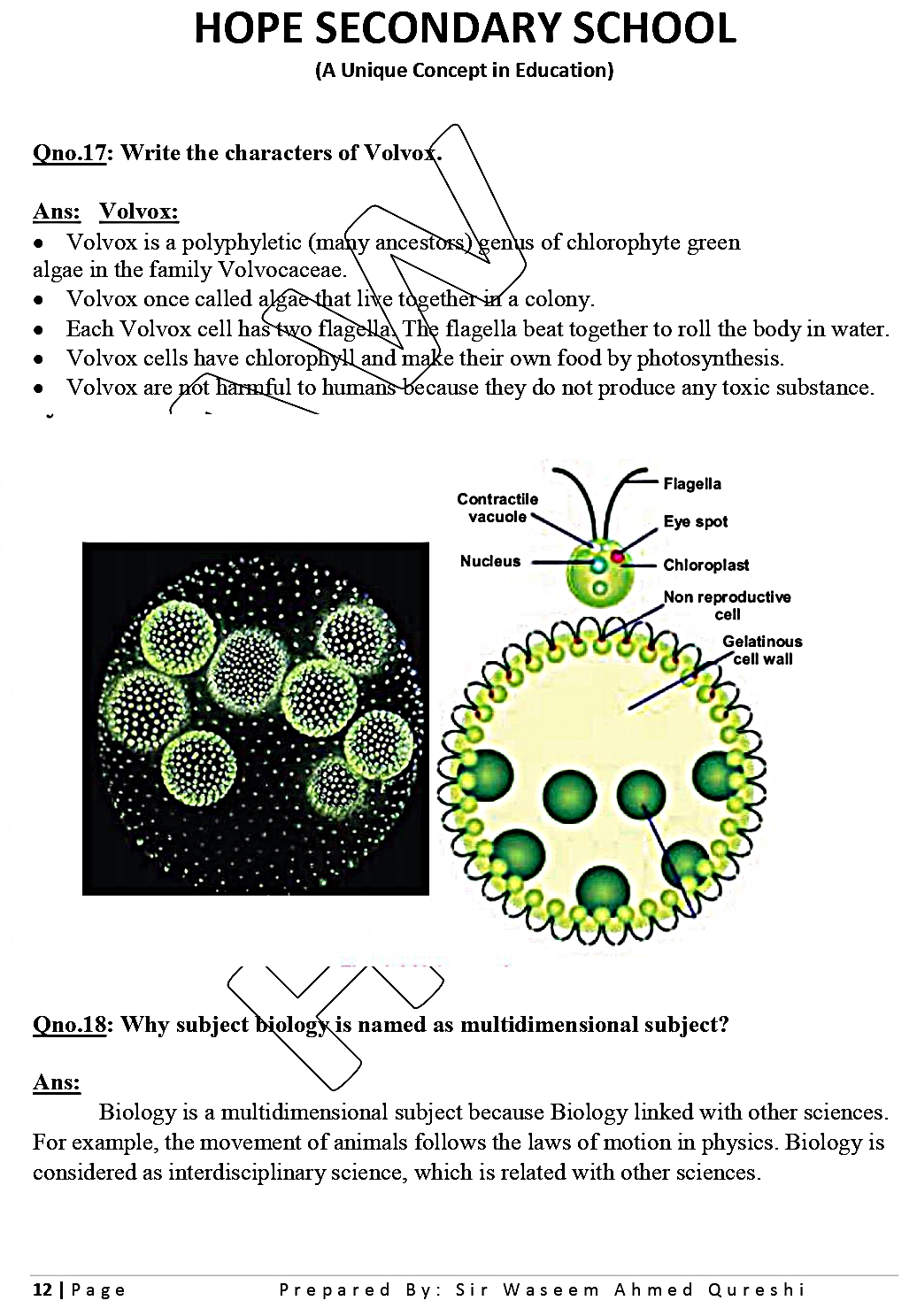
Meal Management And
Food Preservation (ENGLISH)
Past Paper 2021 (MCQs Only)
For HSC Part 2 - Home Economics Group
For Failure, Improvements, Additional Subjects...
Thursday 9 September 2021
Chemistry For Class IX (New Book ) - Chapter No. 6 - Solutions - Multiple Choice Questions And Fill In The Blanks
GO TO INDEX
Chapter No.6 - Solutions
MCQs And Fill In The Blanks
Text Book Exercise
SECTION- A: MULTIPLE CHOICE QUESTIONSTick Mark (✓) the correct answer
1. An alloy is the homogeneous mixture of:
(a) two solid ✓
(b) two liquid
(c) two gases
(d) solid and liquid
2. A saturated solution of KCl on heating becomes:
(a) unsaturated
(b) supersaturated ✓
(c) diluted
(d) all of these
3. If we dissolve sand into the water, then the mixture is said to be:
(a) solution
(b) suspension ✓
(c) colloids
(d) concentrated solution
4. Solubility is usually expressed in grams of the solute dissolved in ________gram of a solvent.
(a) 10 grams
(b) 100 grams ✓
(b) 500 grams
(d) 1000 grams
5. Example of heterogeneous mixture is:
(a) sugar and water
(b) sand and water ✓
(c ) salt and water
(d) ink and water
6. 2 moles of sodium chloride (NaCl) is equal to:
(a) 123 grams
(b) 135 grams
(c) 158 grams
(d) 117 grams ✓
7. Molarity of a solution which is prepared by dissolving 40 g sodium chloride in 500 cm3 of solution is:
(a) 1.4 M
(b) 1.5 M
(c) 1.33 M
(d) 1.37 M ✓
8. 10% (w/w) sugar solution mean that 10 grams of solute dissolved in:
(a) 90g of water ✓
(b) 95g of water
(c) 100g of water
(d) 105g of water
9. An example of true solution is:
(a) solution of starch
(b) solution of soap
(c) ink in water ✓
(d) tooth paste
10. Which solution contain more water:
(a) 1.0 M
(b) 0.75 M
(c) 0.5 M
(d) 0.25 M ✓
11. When a saturated solution is diluted, it change into:
(a) saturated solution
(b) unsaturated solution ✓
(c) concentrated solution
(d) supersaturated solution
12. Butter is example of solution:
(a) gas-liquid
(b) solid-liquid
(c) liquid-solid ✓
(d) gas-solid
13. A solution that contain solid solute into liquid solvent is called:
(a) solids in gas
(b) liquids in solids
(c) solids in solids
(d) solids in liquid ✓
14. What is the particle size in suspension?
(a) 103 nm
(b) 102 nm
(c) less than 103nm
(d) greater than 103 nm ✓
15. Which of the following statements is true?
(a) A solvent is the homogenous mixture of solute and solution.
(b) A solvent is the heterogenous mixture of solute and solution.
(c) A solution is the homogenous mixture of solute and solvent. ✓
(d) A solution is the heterogenous mixture of solute and solvent.
16. Glass of milk, blood and tap water are the examples of:
(a) solution (Colloid) ✓
(b) solute
(c) solvent
(d) None of them
17. The nutrient absorbed by plants from the soil is the example of the:
(a) solvent
(b) solution ✓
(c) Both 'a' & 'b'
(d) None of these
18. Brass is an example of:
(a) solute
(b) solvent
(c) solution (solid) ✓
(d) None of these
19. Brass is:
(a) zinc dissolved in copper ✓
(b) iron dissolved in copper
(c) iron dissolved in zinc
(d) tin dissolved in iron
20. In aqueous solution, water is present in greater amount and termed as:
(a) solution
(b) solvent ✓
(c) solute
(d) None of these
21. The component of a solution which is always present in smaller amount is called the:
(a) solution
(b) solvent
(c) solute ✓
(d) None of these
22. The component of the solution which is present in larger amount is called:
(a) solution
(b) solvent ✓
(c) solute
(d) None of these
23. It is also called as the universal solvent.
(a) Alcohol
(b) Kerosene oil
(c) Mineral oil
(d) Water ✓
24. A solution that cannot dissolve more solute in it at a particular temperature is called a:
(a) saturated solution ✓
(b) unsaturated solution
(c) supersaturated solution
(d) diluted solution
25. A solution that can dissolve more solute than it contained in the saturated solution after heating is called a:
(a) saturated solution
(b) unsaturated solution
(c) supersaturated solution ✓
(d) diluted solution
26. In carbonated drinks, the state of solute is:
(a) gas ✓
(b) liquid
(c) solid
(d) All of these
27. In fog, the state of solvent is:
(a) gas ✓
(b) liquid
(c) solid
(d) All of these
28. Concentration = ____:
(a) mass of solute in gram x volume of solution in dm3
(b) Mass of solute in gram / volume of solution in dm3 ✓
(c) volume of solution in dm3 / Mass of solute in gram
(d) None of these
29. 1 litre = ___:
(a) 1 cm3
(b) 1 ml
(c) 1000 cm3
(d) 1 dm3 ✓
30. Molarity is defined as the number of moles of solute dissolved in:
(a) 1 ml
(b) 1 cm3
(c) 1 dm3 ✓
(d) 1000 cm3
31. Solubility is increased by:
(a) increasing the temperature ✓
(b) decreasing the temperature
(c) leaving the solution for a period of time
(d) All of these
32. 2 moles of water is equal to:
(a) 18 g
(b) 36 g ✓
(c) 56 g
(d) 46 g
33. A solution that contains large amount of dissolved solute is called:
(a) dilute
(b) saturated
(c) supersaturated
(d) concentrated ✓
34. Homogenous mixture of solute and solvent is called:
(a) solvent
(b) solute
(c) solution ✓
(d) suspension
35. The number of ways of representing percent concentration are:
(a) one
(b) two
(c) three
(d) four ✓
36. Solubility is usually expressed in grams of solute per:
(a) 10 grams of solvent
(b) 100 grams of solvent ✓
(c) 1000 grams of solvent
(d) 10000 grams of solvent
37. In colloids, particles are too big to:
(a) dissolve ✓
(b) absorb
(c) crystallize
(d) disappear
38. Suspensions are:
(a) homogenous
(b) heterogeneous ✓
(c) uniform
(d) solutions
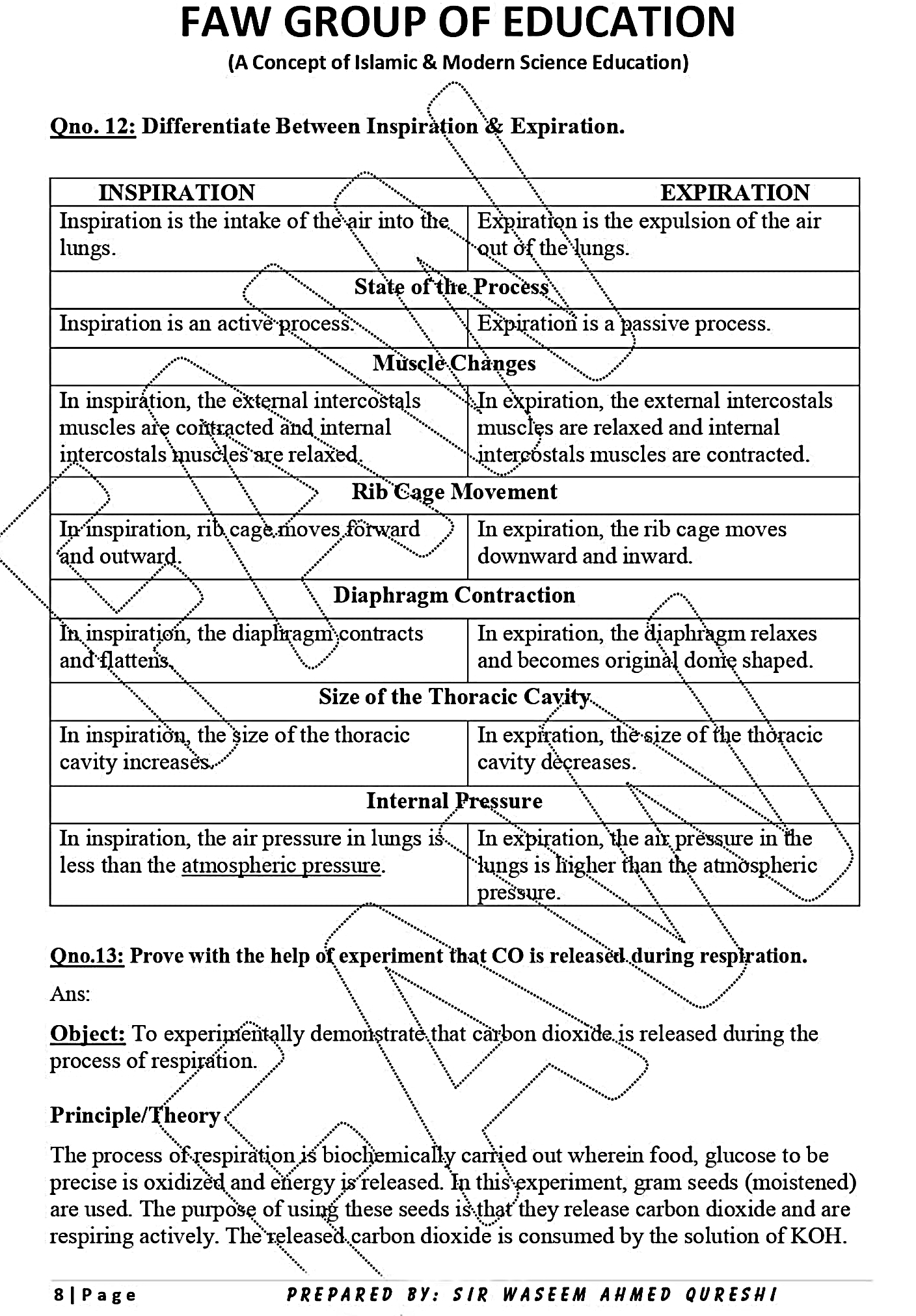
15. Write the example of each type of solution.
| Solute | Solvent | Example |
|---|---|---|
| Solid | Liquid | Salt in water, Sugar in water |
| Gas | Gas | Air, mixture of hydrogen and helium in water balloon, oxygen in air |
| Solid | Solid | Brass an alloy (Zinc dissolved in copper), Bronze (tin dissolved in copper ) |
| Liquid | Solid | Amalgam, butter, cheese |
| Liquid | Gas | Fog( water vapour in air,) liquid air pollutants |
| Liquid | Liquid | Alcohol dissolve sin water, oil in ether. |
Tuesday 7 September 2021
Chemistry Practicals For HSC-Part 1 (XI -Science Group) - Titration - General Descripttion - DJ Sindh Government College
GO TO INDEX
TITRATION
EQUATIONS:NaOH + HCl ⟶ NaCl + H2O
2KOH + H2SO4 ⟶ K2SO4 + 2H2O
KOH + HNO3 ⟶ KNO3 + H2O
2NaOH + H2C204.2H20 ⟶ Na2C204 + 4H20
2KOH + H2C204.2H20 ⟶ 2C204 + 4H20
2HCI + Na2CO3 ⟶ 2NaCI + CO2 + H2O
H2SO4 + K2CO3 ⟶ K2SO4 + CO2 + 2H20
2HNO3 + K2CO3 ⟶ 2KNO3 + CO2 + H2O
H2SO4 + Na2CO3 ⟶ Na2SO4 + CO2 + H2O
2KMn04 + 3H2SO4 ⟶ K2SO4 + 3H20 + 2MnSO4 + 5[O]
10FeSO4 + 5H2SO4 + 5[0] ⟶ 5Fe2(SO4)3 + 5H20
2KMn04 + 10FeSO4 + 8H2SO4 ⟶ K2SO4 + 2MnSO4 + 5Fe2(SO4)3 + 8H20
2KMn04 + 3H2SO4 ⟶ K2SO4 + 3H20 + 2MnSO4 + 5[O]
5H2C2O4 + 5[0] ⟶ 10CO2 + 5H20
2KMn04 + 5H2C2O4 + 3H2SO4 ⟶ K2SO4 + 2MnSO4 + 10CO2 + 8H20
IONIC EQUATION FOR ACID — BASE
Na+ + OH- + H+ + Cl- ⟶ Na+ + Cl- + H2O
2K+ + 20H- + 2H+ + SO42- ⟶ 2K+ + SO42- + 2H20
K+ + OH- + H+ + NO3- ⟶ K+ + NO3- + H20
2Na+ + 20H- + 2H+ + C2042- ⟶ 2Na+ + C2042- + 2H20
2K+ + OH- + 2H+ + C2042- ⟶ 2K+ + C2042- + 2H20
2H+ + 2Cl- + 2Na+ + CO32- ⟶ 2Na+ + 2Cl- + CO2 + H2O
2H+ + SO42- + 2K+ + CO32- ⟶ 2K+ + SO42- + CO2 + H2O
2H+ + 2NO3- + 2K+ + CO32- ⟶ 2K+ + 2NO3- + CO2 + H20
2H+ + SO42- + 2Na+ + CO32- ⟶ 2Na+ + SO42- + CO2 + H2O
THEORY
FOR ACID-BASE TITRATION:
Titration is a process in which the fixed volume of a solution taken by means of pipette into conical flask is compared with the volume of solution added from burette upto end point which is obtained by colour change. The purpose of titration is to determine the unknown strength of a solution.
Base is a substance which gives OH- ion in aqueous solution. When an acid solution reacts completely with base solution or vice versa, a salt is formed.
Neutralization: In acid-base reaction H+ ion from an acid and OH- ion from a base form H2O, salt is also formed process is called neutralization. This is indicated by end point and is observe by suitable indicator those having different colors in different medium.
Strong acid and strong base ionize completely in aqueous solution while weak acid and weak base ionize only partially in aqueous solution, these form acidic and basic salts which hydrolyse to change the pH.
Na2CO3 + H2O ⟶ NaOH + H2CO3
(Basic salt) + (water) (Strong base) (Weak acid)
where Basic (pH > 7)
FOR REDOX TITRATION:
Titration is a process in which the fixed volume of a solution taken by means of pipette Into conical flask is compared with the volume of solution added from burette upto end point which is obtained by colors change. The purpose of titration is to determine the unknown strength of a solution using standard solution.
Redox Titration: The type of titration in which one reactant in oxidized and other reduced are know as redox titration.
Oxidation is a process in which reducing Agent loses electron and absorbs oxygen, so the oxidation number increases.
Fe2+ ⟶ Fe3+ + e-
2FeSO4 + H2SO4 + [0] ⟶ Fe2(SO4)3 + H2O
Reduction is a process in which oxidizing agent gain electron and loses oxygen, so oxidation number decreases Mn7+ + 5e- ⟶ Mn2+
2KMnO4 + H2SO4 ⟶ K2SO4 2MnSO4 + 3H20 + 5[O]
Oxidizing strength of KMnO4 increase in the presence of H2SO4, so H2SO4 is required in redox titration. KMnO4 is coloured substance so it acts as indicator, therefore no external indicator is required.Reaction between KMnO4 and oxalic acid is very slow because oxalic acid is a covalent organic compound. So the Titration flask is heated to about 60 — 70°C before adding KMnO4 from the burette.
PROCEDURE:
Strong Base Vs Stron Acid OR Strong Base Vs Weak Acid
1. Wash burette, pipette and conical flask with water.
2. Rinse burette with base and pipette with acid.
3. Do not rinse the conical flask.
4. Fill the burette with base upto Zero mark.
5. Pipette out 10 cm3 of acid into the conical flask.
6. Add one or two drops of phenolphthalein as indicator.
7. Titrate the base in the burette with the acid in conical flask. The burette solution is added drop by drop into the conical flask.
8. Shake the flask after each addition, The end point is indicated by colour change from colourless to light pink.
9. Note the end point.
10. wash the conical flask with water and repeats the same process for at least three times to obtain two similar readings.
Strong Acid Vs Weak Base
1. Wash burette, pipette and conical flask with water.
2. Rinse burette with acid and pipette with base solution.
3. Do not rinse the conical flask.
4. Fill the burette with acid upto Zero mark.
5. Pipette out 10 cm3 of base into the conical flask.
6. Add one or two drops of methyl orange as indicator.
7. Titrate the acid in the burette with the base in conical flask. The burette solution is added drop by drop into the conical flask.
8. Shake the flask after each addition, The end point is indicated by colour change from yellow to light pink.
9. Note the end point.
10. Wash the conical flask with water and repeat the same process for at least three times to obtain two similar readings.
Redox Titration
1. Wash all the apparatus with water.
2. Rinse burette with KMnO4 and pipette with reducing agent.
3. Do not rinse the conical flask.
4. Fill the burette with base upto Zero mark.
5. Pipette out 10 cm3 of given reducing agent into the conical flask.
6. Add one test tube of dilute H2SO4 (In case of oxalic acid as a reducing agent titration flask is heated to about 60 - 70°C before titration).
7. Titrate KMnO4 in the burette with the reducing agent in conical flask. The burette solution is added drop by drop into the conical flask.
8. Shake the flask after each addition, the end point is indicated by colour change from colourless to light pink.
9. Note the end point.
10. Wash the conical flask with water repeat the same process for at least three times to obtain two similar readings.
OBSERVATION:
| S.NO. | Initial reading | Final reading | Difference | Concordant reading |
|---|---|---|---|---|
| 1. | cm3 | cm3 | cm3 | cm3 |
| 2. | cm3 | cm3 | cm3 | |
| 3. | cm3 | cm3 | cm3 |
- Solution in burette = ______ (Strong Acid Or Strong Base)
- Solution in conical flask = ________ (Weak Acid Or Weak Base)
- Indicator used = _______ (e.g Phenophthalein for Strong Base)
- Normality of standard solution = N1 = ___ N
- Volume of standard solution = V1 = 10 ml
- Normality of solution of unknown strength = N2 = ?
- Volume of solution of unknown strength = V2 = ?
CALCULATION:
Standard solution Vs Solution of unknown strength
N1V1 = N2V2
Equivalent mass = Molecular mass / Acidity or Basicity or change in oxidation state Amount = Normality (N2) x equivalent mass x volume of solution in cm3 / 1000
RESULT:
- Normality of given ________ solution = ______ N
- Amount of ______ in the given solution = gms / ___ dm3 or ____ cm3
Acidity Of Base:
| Base | Acidity | Equivalent mass |
|---|---|---|
| NaOH | 1 | 40 |
| KOH | 1 | 56 |
| Na2CO3 | 2 | 53 |
| K2CO3 | 2 | 69 |
Basicity Of Acid
| Acid | Basicity | Equivalent mass |
|---|---|---|
| HCl | 1 | 36.5 |
| H2SO4 | 2 | 49 |
| HNO3 | 1 | 63 |
| C2H204.2H20 | 2 | 63 |
Oxidizing Agent
| Oxidizing agent | No. of electron gained | Equivalent mass |
|---|---|---|
| KMnO4 | 5 | 31.6 |
Reducing Agent
| Reducing Agent | No. of electron lost | Equivalent mass |
|---|---|---|
| FeSO4.7H20 | 1 | 278 |
| FeSO4.(NH4)2SO4.6H20 | 1 | 392 |
| H2C204.2H20 | 2 | 63 |
Monday 6 September 2021
Biology For Class IX - Chapter No.1 - Introduction To Biology - Notes By FAW Group Of Education - Hope Secondary School
GO TO INDEX
CHAPTER 1
INTRODUCTION TO BIOLOGY
Notes By FAW - Hope Secondary School
Special Thanks To Sir Waseem Ahmed Qureshi
Sunday 5 September 2021
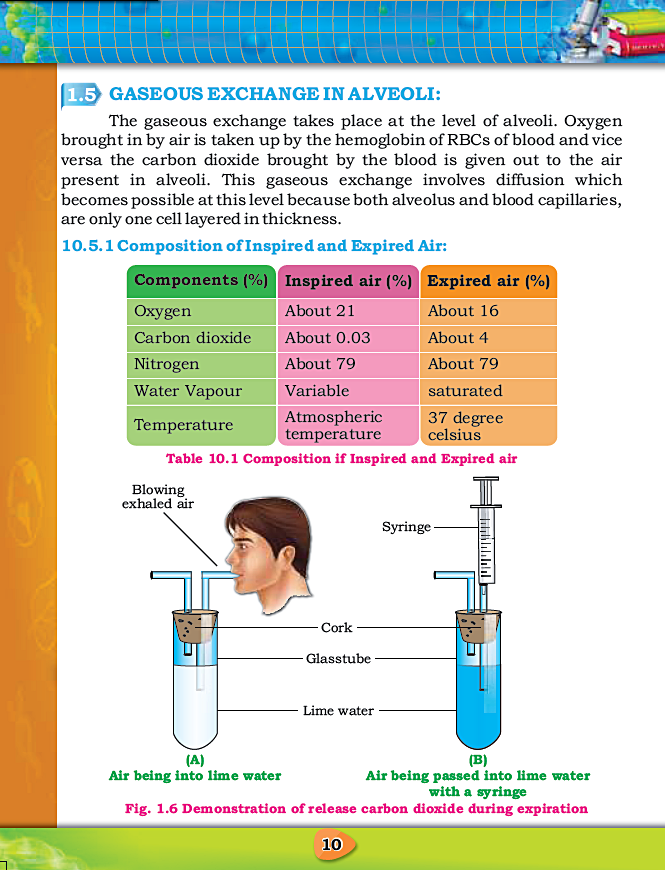
Biology For Class X - Chapter No. 1 - Gaseous Exchange - Notes By FAW Group Of Education
GO TO INDEX
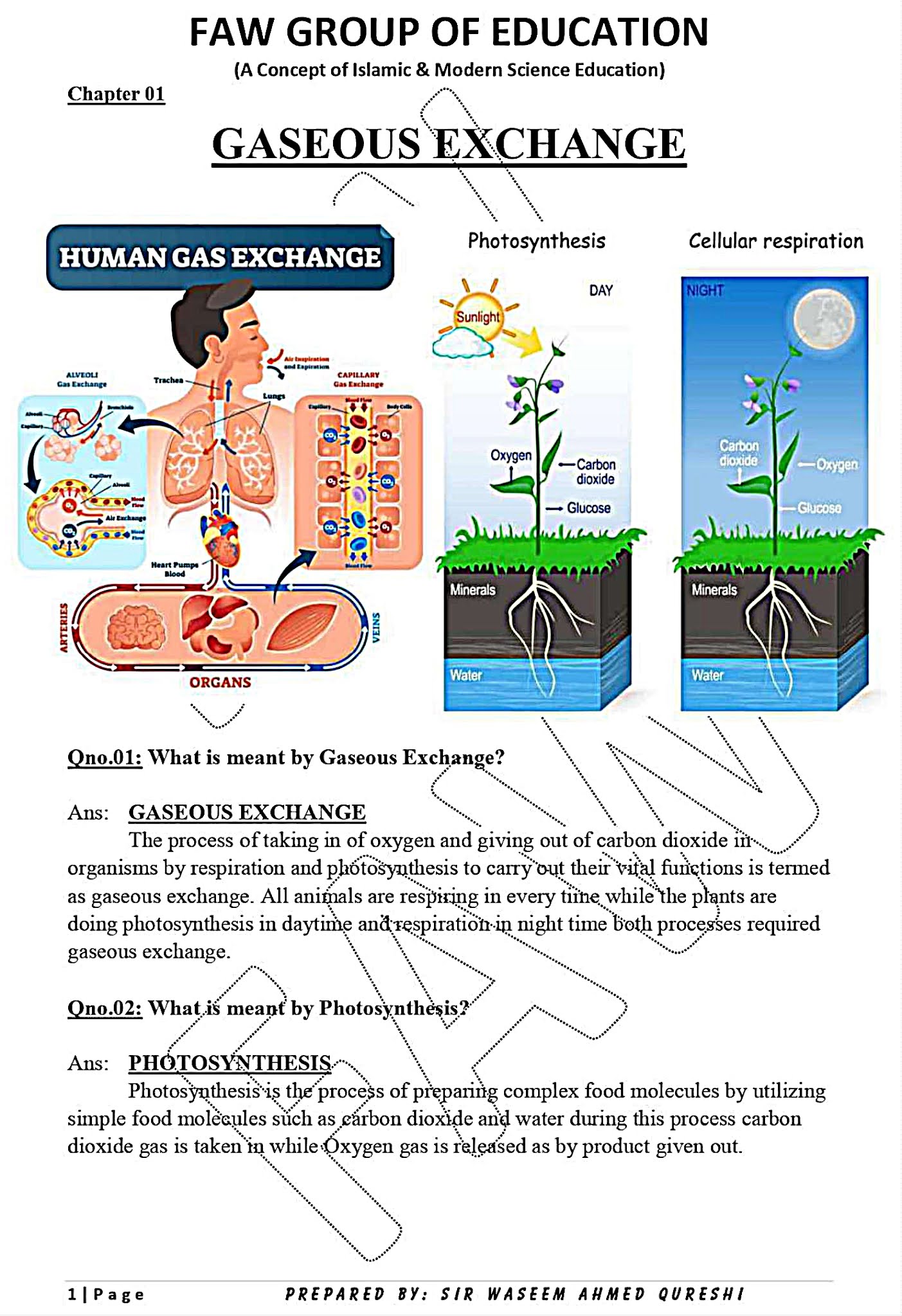
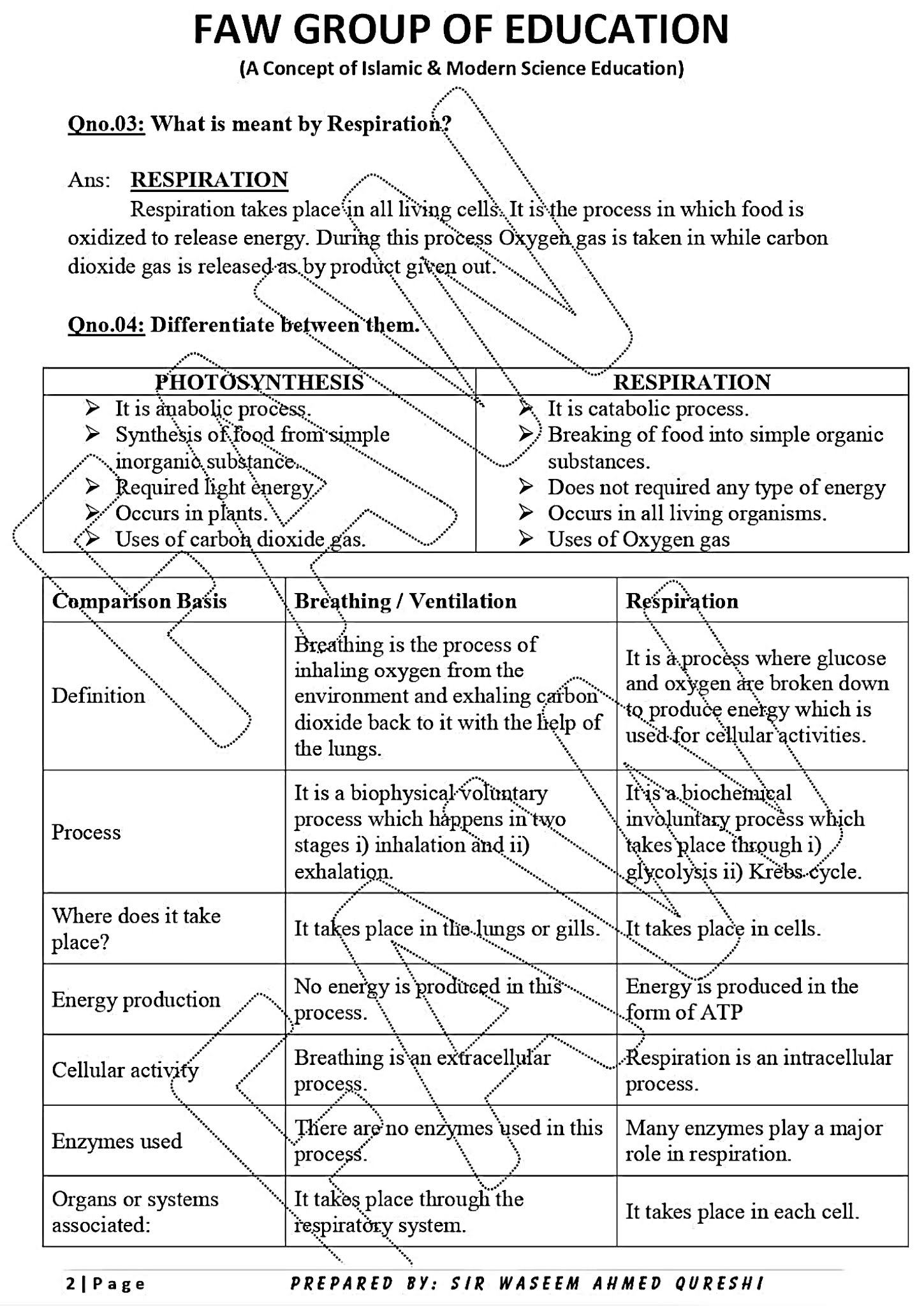
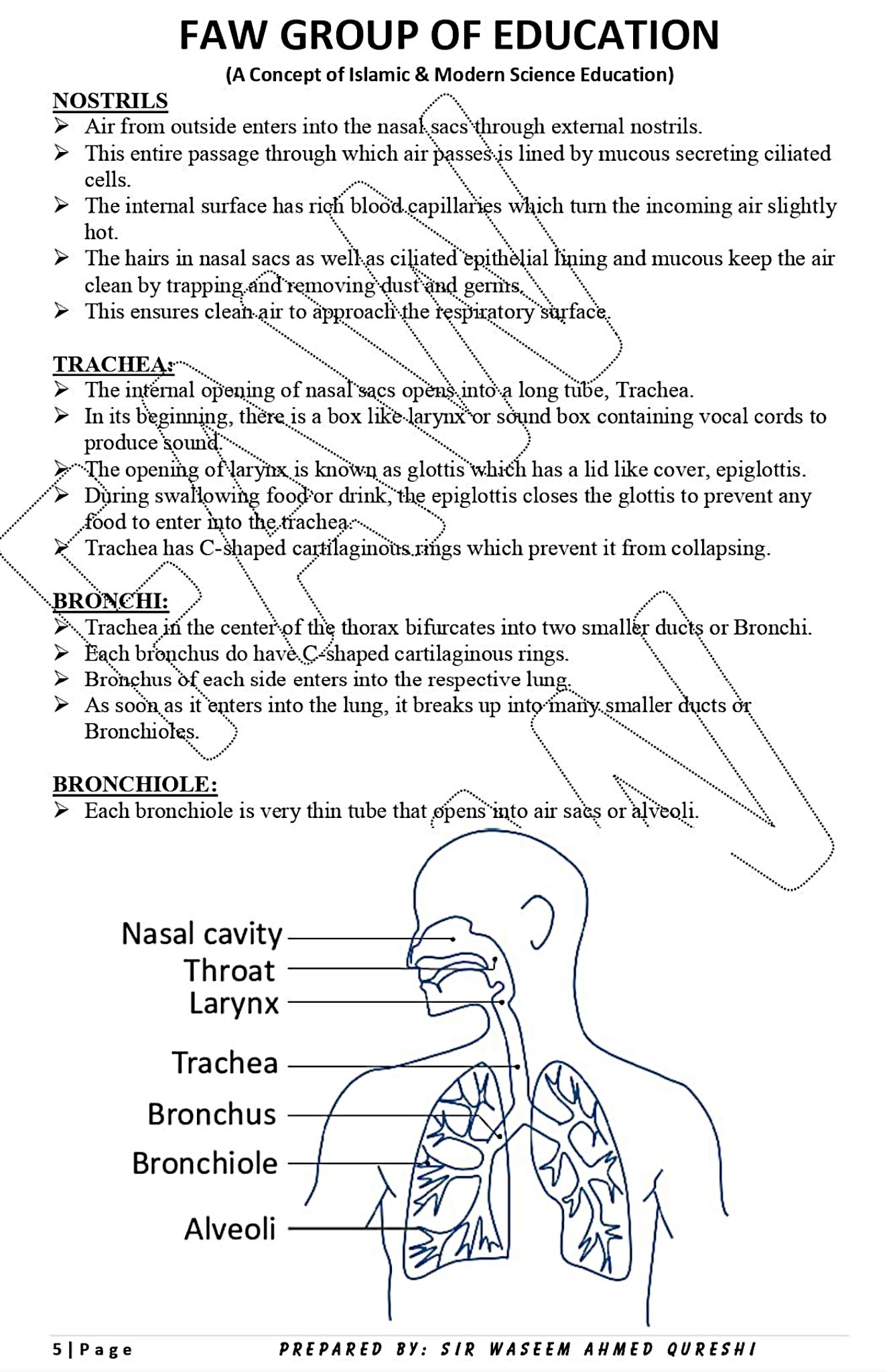
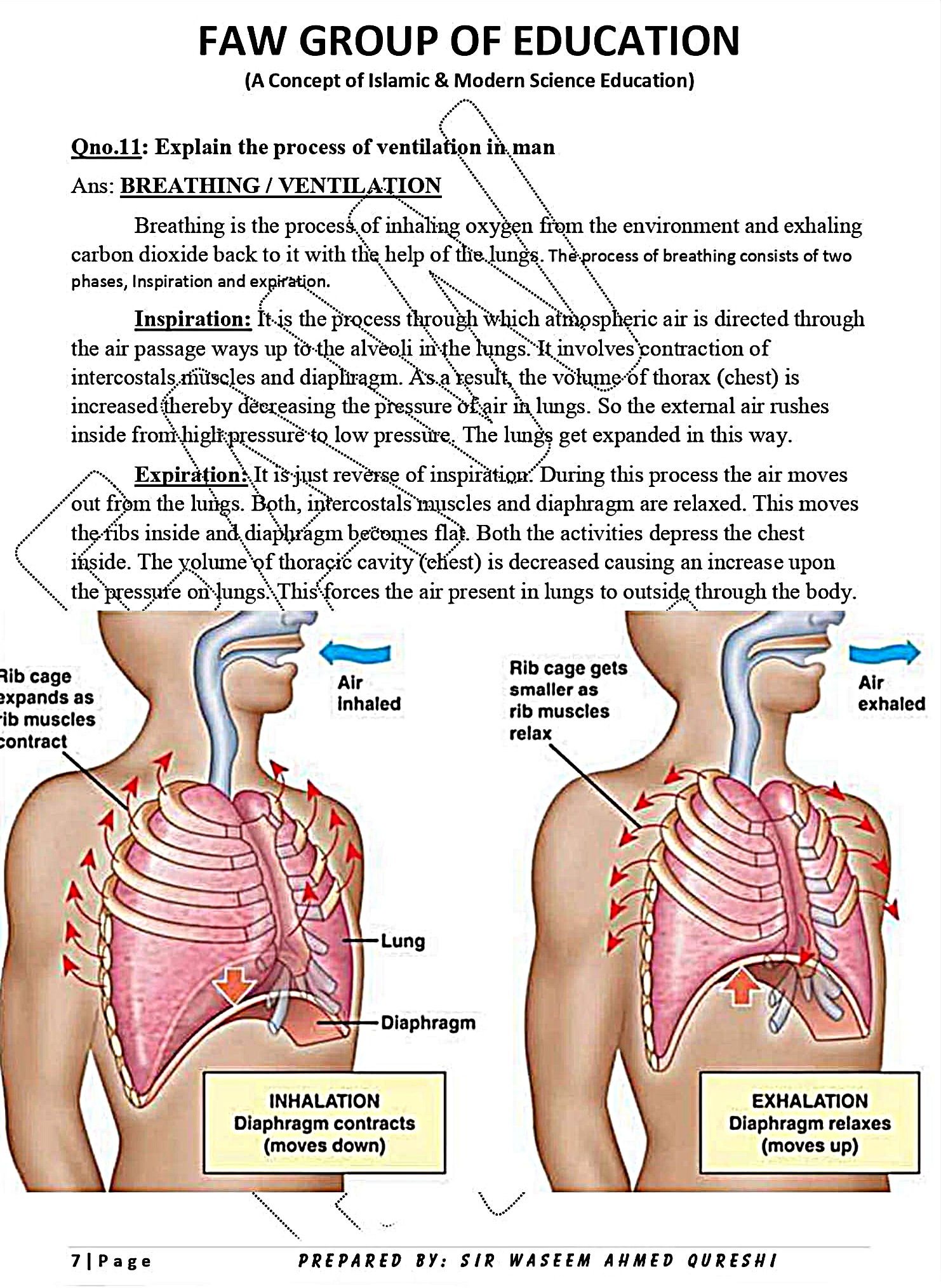
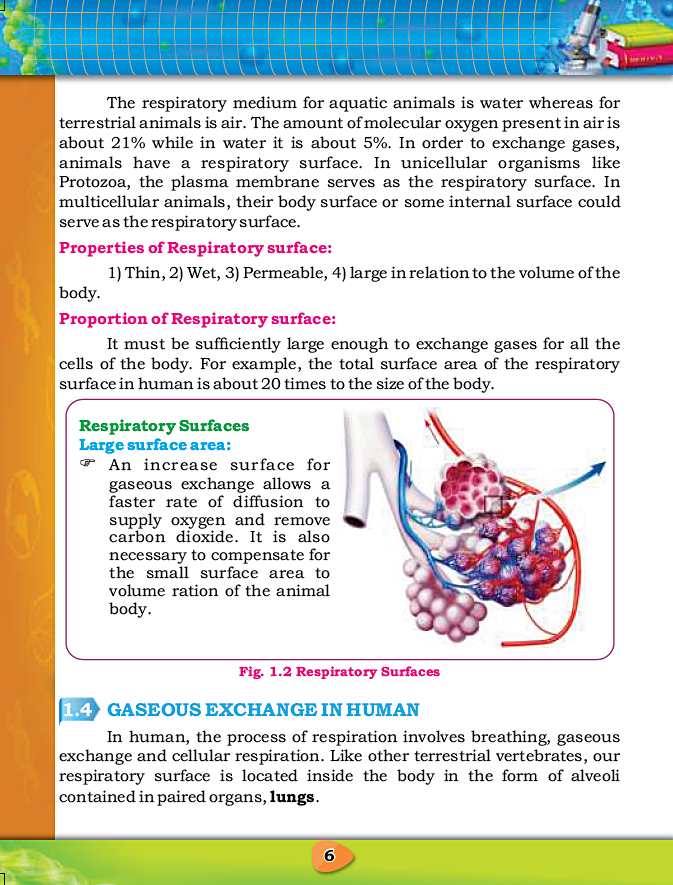
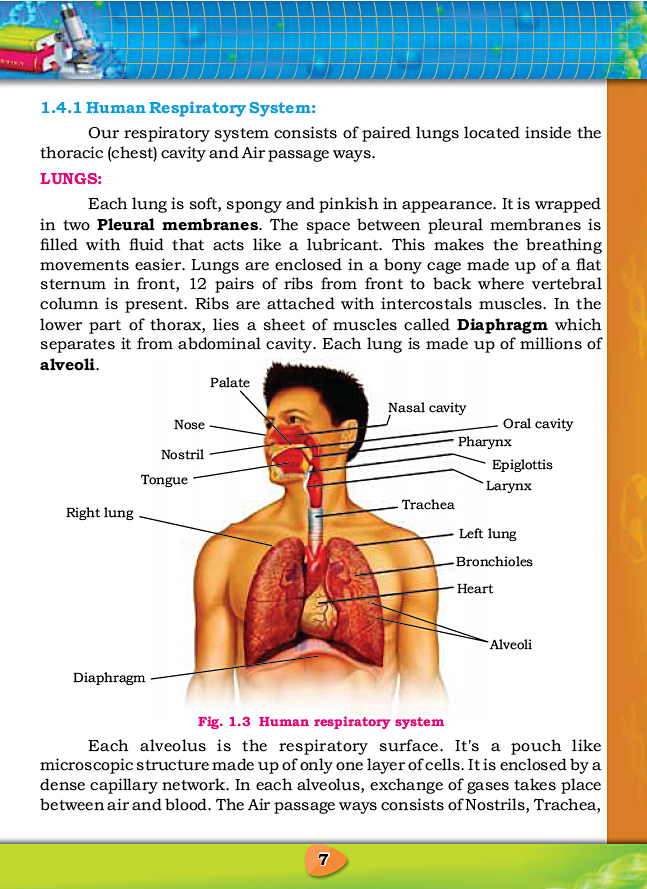
CHAPTER 1: GASEOUS EXCHANGE
Notes By FAW Group Of Education
(VIDEO LESTURES ARE ALSO AVAILABLE ON WHATSAPP 0315-0226373)
KARACHI GRAMMAR SCHOOL
(Plot No. 39-40, Bismillah Chowk, Saeedabad Baldia Town, Karachi)
PREPARED BY SIR WASEEM AHMED QURESHI (BS ZOOLOGY)(Plot No. 39-40, Bismillah Chowk, Saeedabad Baldia Town, Karachi)
For Educational News update like our FACEBOOK Page and YOUTUBE Channel:
Face Book : FAW Educational & Skills Institute
You Tube : FAW Group of Education
Whatapp : Sir Waseem Ahmed Qureshi: 03150226373 (Any Comment OR Question about Biology Notes Contact on Whatsapp)
Subscribe to:
Posts (Atom)