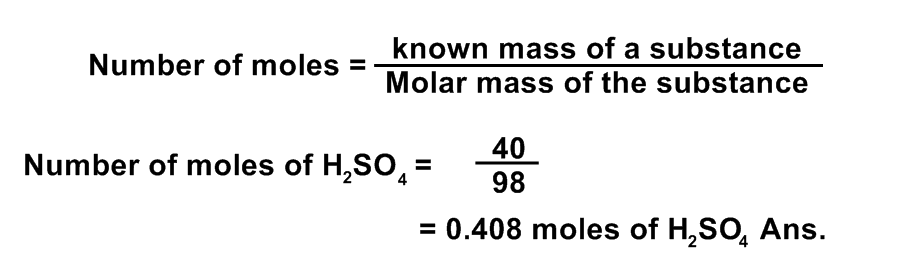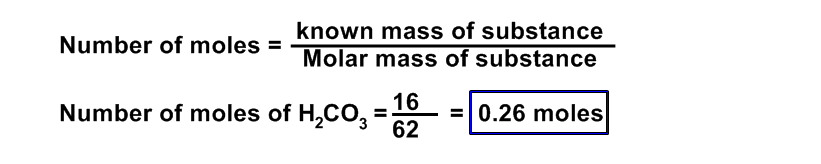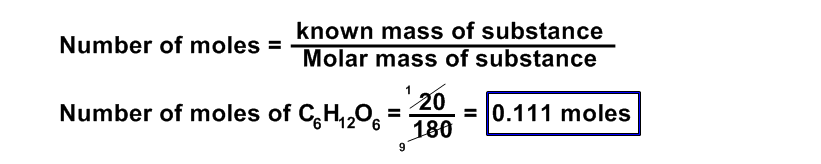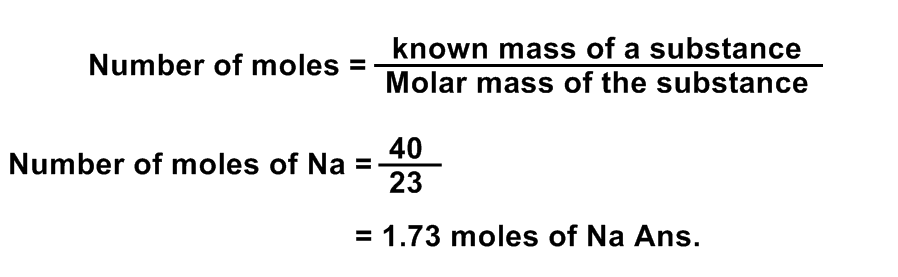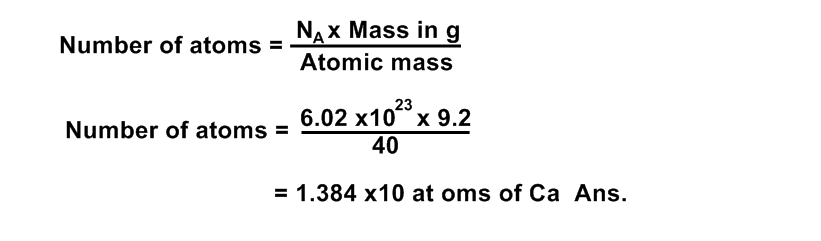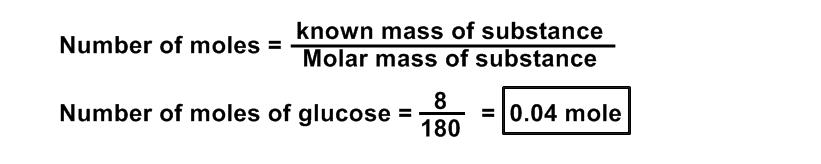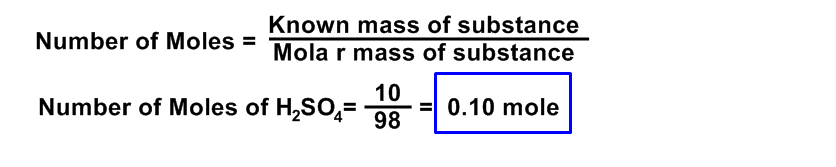Search This Blog
Tuesday 14 September 2021
Family Clothing Problems (Ur) - Past Paper 2021 (MCQs Only) - For HSC Part 1 (Home Economics Group) - For Failure, Improvements, Additional Subjects...
GO TO INDEX
Family Clothing Problems (Ur)
Past Paper 2021 (MCQs Only)
For HSC Part 1
(Home Economics Group)
For Failure, Improvements, Additional Subjects...
Family Clothing Problems (Eng) - Past Paper 2021 (MCQs Only) - For HSC Part 1 (Home Economics Group) - For Failure, Improvements, Additional Subjects...
GO TO INDEX
Family Clothing Problems (Eng)
Past Paper 2021 (MCQs Only)
For HSC Part 1
(Home Economics Group)
For Failure, Improvements, Additional Subjects...
Family Relations And Child Development (Eng And Ur) - Past Paper 2021 (MCQs Only) - For HSC Part 1 (Home Economics Group) - For Failure, Improvements, Additional Subjects...
GO TO INDEX
Family Relations And Child Development
(Eng And Ur)
Past Paper 2021 (MCQs Only)
For HSC Part 1
(Home Economics Group)
For Failure, Improvements, Additional Subjects...
Chemistry Practicals For HSC-Part 1 (XI -Science Group) - Experiment No.2 : Titration (KOH - H2SO4)
GO TO INDEX
TITRATION
By D. J. Sindh Govt. Science CollegeOBJECT:
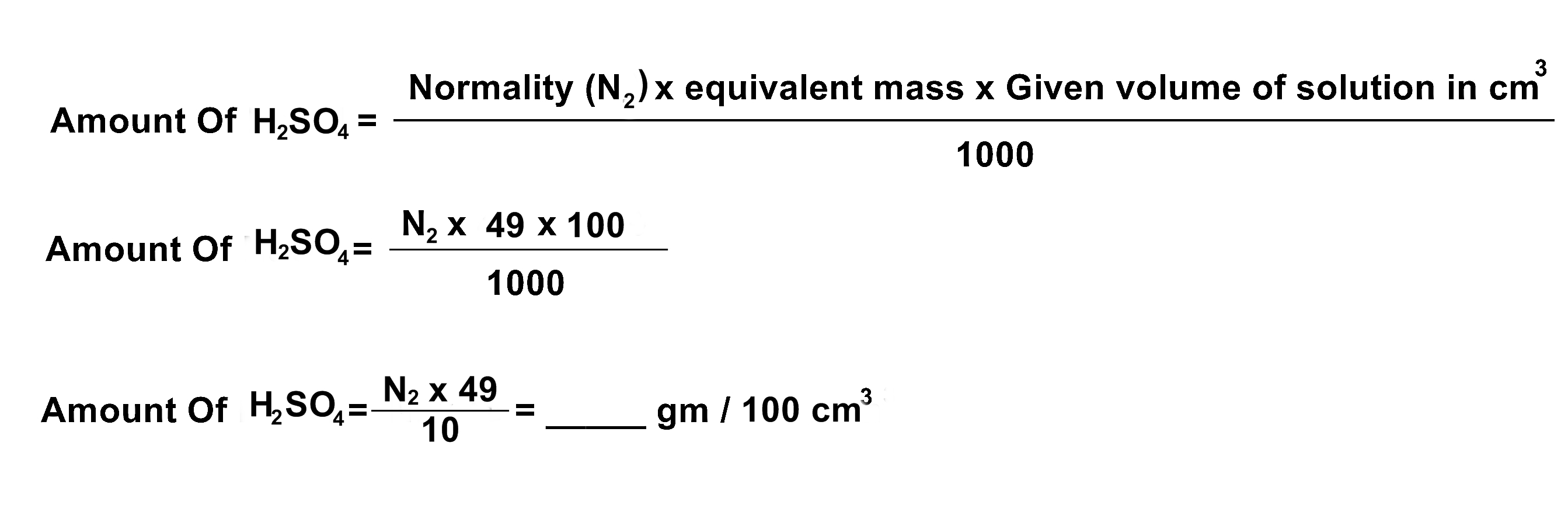
(2) You are given decinormal (1/ 10 or 0.1 N) solution of KOH. Find out Normality and amount of given H2SO4 per 100 cm3 solution.
APPARATUS:
- Burette
- Pipette
- Conical Flask
- Burette Stand with clamp
- Dropper
THEORY:
ACID-BASE TITRATION:
Titration is a process in which the fixed volume of a solution taken by means of pipette into conical flask is compared with the volume of solution added from burette upto end point which is obtained by colour change. The purpose of titration is to determine the unknown strength of a solution.
Base is a substance which gives OH- ion in aqueous solution. When an acid solution reacts completely with base solution or vice versa, a salt is formed.
Neutralization: In acid-base reaction H+ ion from an acid and OH- ion from a base form H2O, salt is also formed process is called neutralization. This is indicated by end point and is observe by suitable indicator those having different colors in different medium.
Strong acid and strong base ionize completely in aqueous solution while weak acid and weak base ionize only partially in aqueous solution, these form acidic and basic salts which hydrolyse to change the pH.
Na2CO3 + H2O ⟶ NaOH + H2CO3
(Basic salt) + (water) (Strong base) (Weak acid)
where Basic (pH > 7)
EQUATIONS
2KOH + H2SO4 ⟶ K2SO4 + 2H2O
IONIC EQUATION
2K+ + 20H- + 2H+ + SO42- ⟶ 2K+ + SO42- + 2H20
PROCEDURE:
Strong Base Vs Stron Acid
- Wash burette, pipette and conical flask with water.
- Rinse burette with base (KOH) and pipette with acid (H2SO4).
- Do not rinse the conical flask.
- Fill the burette with base (KOH) upto Zero mark.
- Pipette out 10 cm3 of acid (H2SO4) into the conical flask.
- Add one or two drops of phenolphthalein as indicator.
- Titrate the base in the burette with the acid in conical flask. The burette solution is added drop by drop into the conical flask.
- Shake the flask after each addition, The end point is indicated by colour change from colourless to light pink.
- Note the end point.
- Wash the conical flask with water and repeats the same process for at least three times to obtain two similar readings.
OBSERVATION:
Volume Of NaOH = V1 = ___ cm3
| S.NO. | Initial reading | Final reading | Difference | Concordant reading |
|---|---|---|---|---|
| 1. | cm3 | cm3 | cm3 | ___ cm3 |
| 2. | cm3 | cm3 | cm3 | |
| 3. | cm3 | cm3 | cm3 |
- Solution in burette = KOH
- Solution in conical flask = H2SO4
- Indicator used = Phenophthalein
- Normality of standard solution Of KOH = N1 = 0.1 N
- Volume of KOH solution = V1 = _____ ml (Hint: Burette - concordant reading)
- Normality of H2SO4 solution = N2 = ___ N
- Volume of standard solution Of H2SO4 = V2 = 10 ml
- Amount of H2SO4 = _____ gms/cm3
CALCULATION:
Normality Of H2SO4
N1 (base) x V1(base) = N2 (acid) x V2 (acid)
OR
OR
N2 = (N1 x V1) / V2 = ____ N
Equivalent mass Of H2SO4 = Molecular mass / Basicityty of H2SO4
Molecular mass of H2SO4 = 2(1) + 1(32) + 4(16) = 2 + 32 + 64 = 98 a.m.u
Equivalent mass Of H2SO4 =
Amount Of H2SO4 In gms / 100 cm3
RESULT:
Normality of given H2SO4 solution = ______ N
Amount of H2SO4 in the given solution = ____ gms / 100 cm3
OR
By Mubarka Naz
EXPERIMENT NO.2
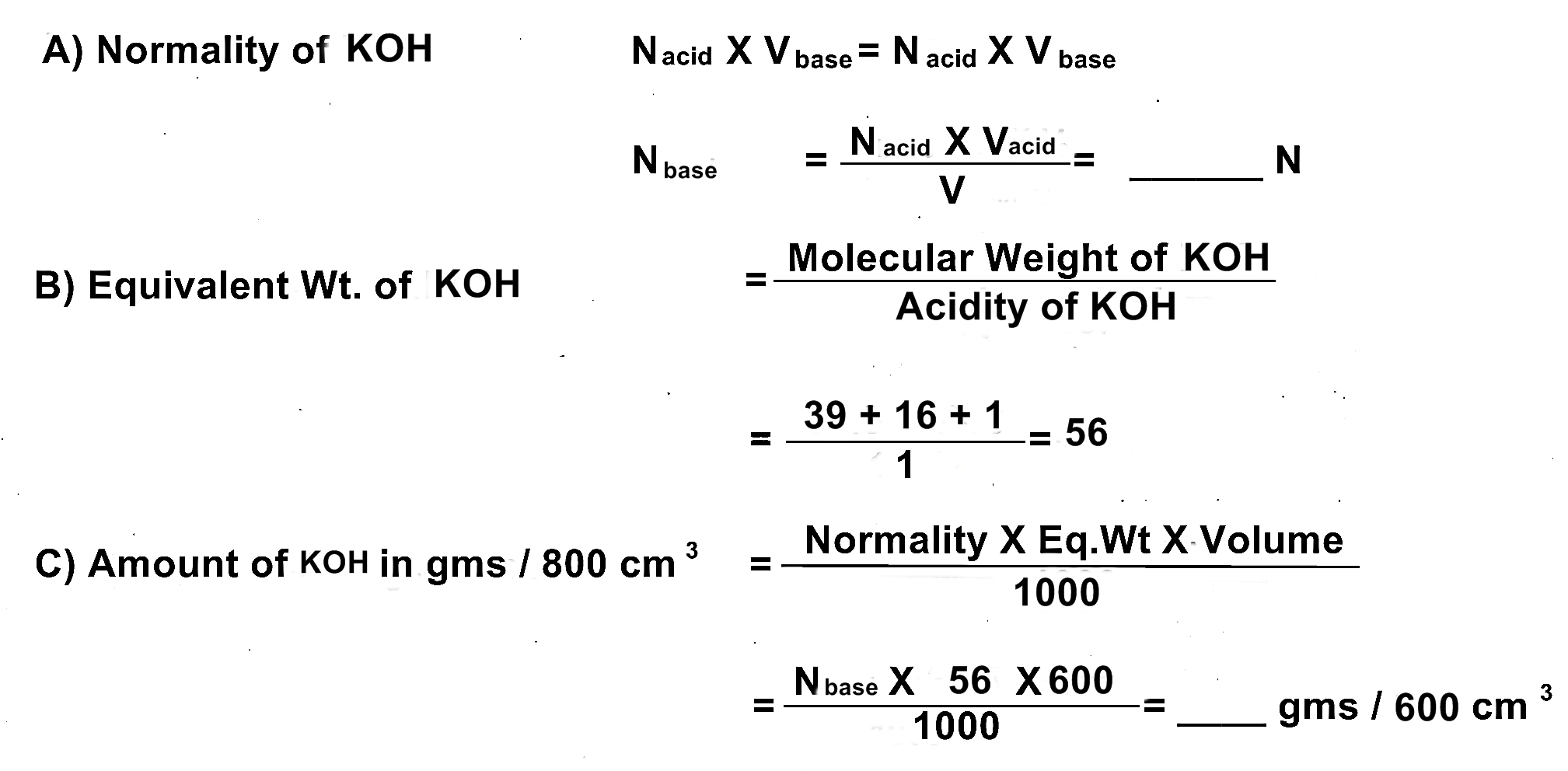
OBJECT: You are provided N/18 (0.56 N) Solution of H2SO4. Determine the normality and amount of KOH in gms per 600 cm3.
APPARATUS:
- Burette
- Pipette
- Conical Flask
- Burette Stand
- Clamp with holder
- Dropper
THEORY:
When an acid reacts completely with an equivalent amount of a base neutralization takes place. Neutralization is the process in which H+ ion of an acid and OH- ions of a base combine to form water. A salt is also formed during the neutralization.
Acid + Base ⟶ Water + Salt
EQUATION:
2KOH + H2SO4 ⟶ K2SO4 + 2H2O
IONIC EQUATION:
2K+ + 20H- + 2H+ + SO42- ⟶ 2K+ + SO42- + 2H20
PROCEDURE:
1) Wash the burette pipette and conical flask with water.
2) Rinse the burette with KOH solution and pipette with H2SO4 solution.
3) Fill the burette with KOH solution leaving no air bubble in the jet and clamp it vertically in to the burette stand.
4) Pipette out 10 cm3(ml) of H2SO4 solution in to the conical flask and add 1 - 2 drops of phenolphthalein indicator.
5) Start adding the solution of NaOH drop by drop from the burette into the conical flask, shake the flask after each addition.
6) Continue the addition of KOH solution till a permanent pinkish tinge appears with a single drop of KOH solution, record the reading of burette at this stage.
7) Wash the conical flask with water and repeat the process of titration till concordant reading is obtained.
OBSERVATIONS:
i) Solution in burette: KOH
ii) Solution in conical flask: H2SO4
iii) Normality of Acid: 0.56 N
iv) Normality of base: ? N
v) Indicator: Phenolphthalein
vi) Change of colour: colourless to pink
| OBS Nos. | Initial Reading Of Burette (X cm3) |
End Point Reading Of Burette (Y cm3) |
Volume consume (Y - X cm3) |
|---|---|---|---|
| 1. | 0 | 8.0 | 8.0 |
| 2. | 8.0 | 16.0 | 8.0 |
| 3. | 16.0 | 24.0 | 8.0 |
| 4. |
Concordant Reading = (8.0 + 8.0 + 8.0) / 3 = 8.0 cm3
CALCULATIONS:
RESULT:
Normality of the given KOH solution = ___ N or gram.equivalent/ltr/dm3.
Amount of KOH in the given solution = _____ gms / 600 cm3.
Chemistry Practicals For HSC-Part 1 (XI -Science Group) - Experiment No.1 : Titration (NaOH - HCl)
GO TO INDEX
TITRATION
By D. J. Sindh Govt. Science CollegeOBJECT:
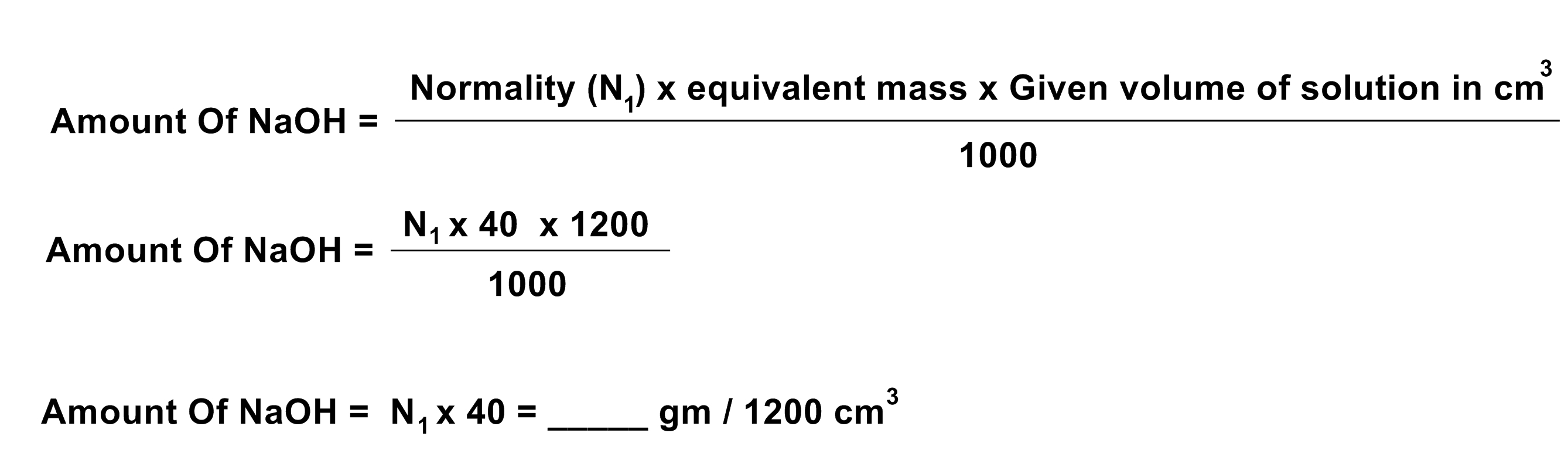
(1) You are given 0.2 N solution of HCl. Find out Normality and amount of given NaOH per 1200 cm3 solution.
APPARATUS:
- Burette
- Pipette
- Conical Flask
- Burette Stand with clamp
- Dropper
THEORY:
ACID-BASE TITRATION:
Titration is a process in which the fixed volume of a solution taken by means of pipette into conical flask is compared with the volume of solution added from burette upto end point which is obtained by colour change. The purpose of titration is to determine the unknown strength of a solution.
Base is a substance which gives OH- ion in aqueous solution. When an acid solution reacts completely with base solution or vice versa, a salt is formed.
Neutralization: In acid-base reaction H+ ion from an acid and OH- ion from a base form H2O, salt is also formed process is called neutralization. This is indicated by end point and is observe by suitable indicator those having different colors in different medium.
Strong acid and strong base ionize completely in aqueous solution while weak acid and weak base ionize only partially in aqueous solution, these form acidic and basic salts which hydrolyse to change the pH.
Na2CO3 + H2O ⟶ NaOH + H2CO3
(Basic salt) + (water) (Strong base) (Weak acid)
where Basic (pH > 7)
EQUATIONS
NaOH + HCl ⟶ NaCl + H2O
IONIC EQUATION
Na+ + OH- + H+ + Cl- ⟶ Na+ + Cl- + H2O
PROCEDURE:
Strong Base Vs Stron Acid
- Wash burette, pipette and conical flask with water.
- Rinse burette with base (NaOH) and pipette with acid (HCl).
- Do not rinse the conical flask.
- Fill the burette with base (NaOH) upto Zero mark.
- Pipette out 10 cm3 of acid (HCl) into the conical flask.
- Add one or two drops of phenolphthalein as indicator.
- Titrate the base in the burette with the acid in conical flask. The burette solution is added drop by drop into the conical flask.
- Shake the flask after each addition, The end point is indicated by colour change from colourless to light pink.
- Note the end point.
- Wash the conical flask with water and repeats the same process for at least three times to obtain two similar readings.
OBSERVATION:
Volume Of NaOH = V1 = ___ cm3
| S.NO. | Initial reading | Final reading | Difference | Concordant reading |
|---|---|---|---|---|
| 1. | cm3 | cm3 | cm3 | ___ cm3 |
| 2. | cm3 | cm3 | cm3 | |
| 3. | cm3 | cm3 | cm3 |
- Solution in burette = NaOH
- Solution in conical flask = HCl
- Indicator used = Phenophthalein
- Normality of standard solution Of NaOH = N1 = ____ N
- Volume of NaOH solution = V1 = _____ ml (Hint: Burette - concordant reading)
- Normality of HCl solution = N2 = 0.2N
- Volume of standard solution Of HCl= V2 = 10 ml
- Amount of NaOH = _____ gms/cm3
CALCULATION:
Normality Of NaOH
N1 x V1 = N2 x V2
OR
OR
N1 = (N2 x V2) / V1 = ____ N
Equivalent mass Of NaOH = Molecular mass / Acidity of NaOH
Equivalent mass Of NaOH = 40 / 1 = 40
Amount Of NaOH In gms / 1200 cm3
RESULT:
Normality of given NaOH solution = ______ N
Amount of NaOH in the given solution = ____ gms / 1200 cm3
OR
By Mubarka Naz
EXPERIMENT NO.1
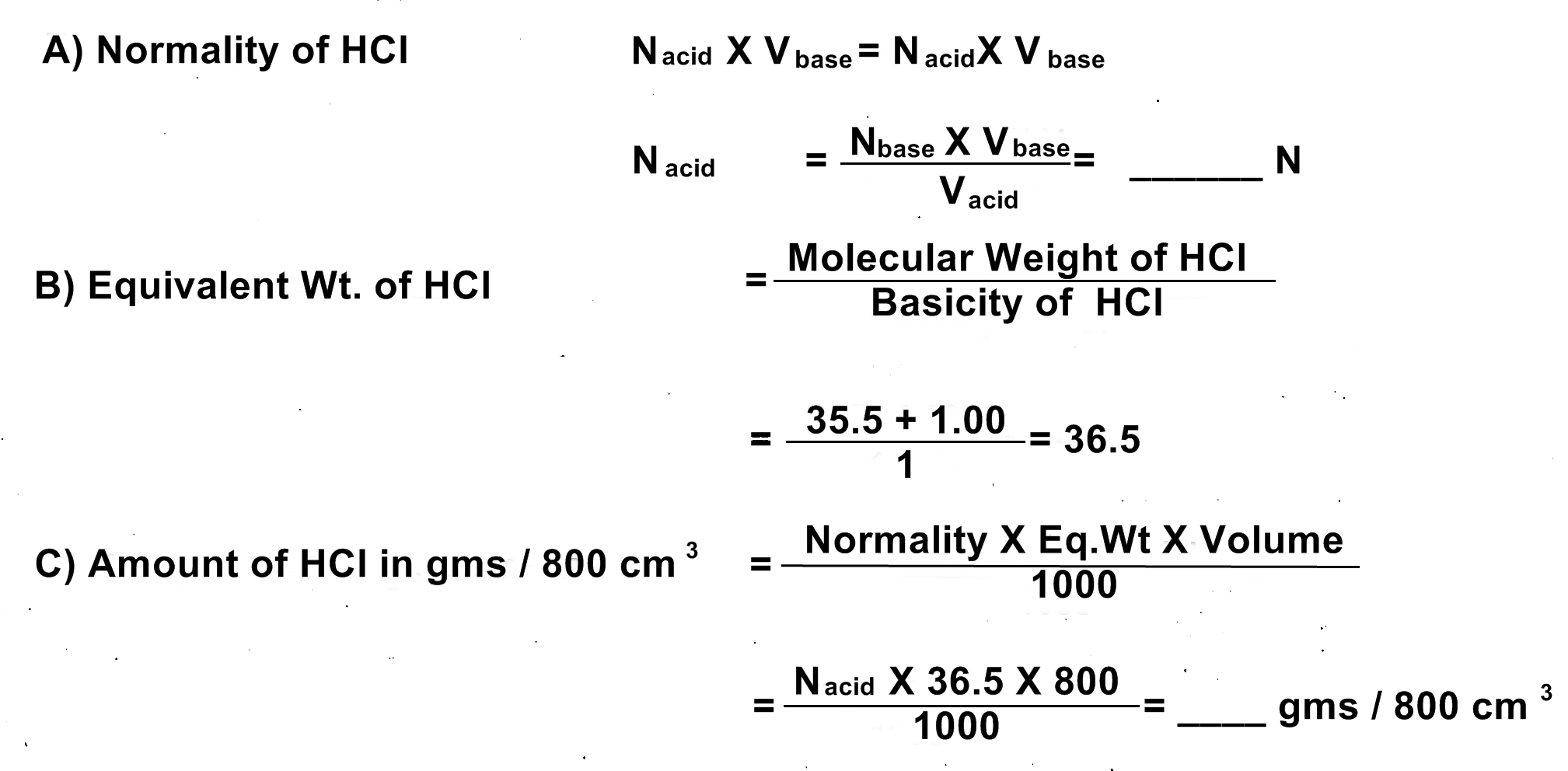
OBJECT: Given 0.1 N Solution of NaOH. Determine the normality and amount of HCl in gms per 800 cm3.
APPARATUS:
- Burette
- Pipette
- Conical Flask
- Burette Stand
- Clamp with holder
- Dropper
THEORY:
When an acid reacts completely with an equivalent amount of a base neutralization takes place. Neutralization is the process in which H+ ion of an acid and OH- ions of a base combine to form water. A salt is also formed during the neutralization.
Acid + Base ⟶ Water + Salt
EQUATION:
HCl + NaOH ⟶ H2O + NaCl
IONIC EQUATION:
H+ + OH- + Na+ + OH- ⟶ H2O + Na+ + Cl-
PROCEDURE:
1) Wash the burette pipette and conical flask with water.
2) Rinse the burette with NaOH solution and pipette with HCl solution.
3) Fill the burette with NaOH solution leaving no air bubble in the jet and clamp it vertically in to the burette stand.
4) Pipette out 10 cm3(ml) of HCl solution in to the conical flask and add 1 - 2 drops of phenolphthalein indicator.
5) Start adding the solution of NaOH drop by drop from the burette into the conical flask, shake the flask after each addition.
6) Continue the addition of NaOH solution till a permanent pinkish tinge appears with a single drop of NaOH solution, record the reading of burette at this stage.
7) Wash the conical flask with water and repeat the process of titration till concordant reading is obtained.
OBSERVATIONS:
i) Solution in burette: NaOH
ii) Solution in conical flask: HCl
iii) Normality of Acid: ?
iv) Normality of base: 0.1 N
v) Indicator: Phenolphthalein
vi) Change of colour: colourless to pink
| OBS Nos. | Initial Reading Of Burette (X cm3) |
End Point Reading Of Burette (Y cm3) |
Volume consume (Y - X cm3) |
|---|---|---|---|
| 1. | 0 | 6.1 | 6.1 |
| 2. | 6.1 | 12.2 | 6.1 |
| 3. | 12.2 | 18.2 | 6.0 |
| 4. | 18.2 | 24.3 | 6.1 |
Concordant Reading = (6.1 + 6.1 + 6.0 + 6.1) / 4 = 6.075 cm3
CALCULATIONS:
RESULT:
Normality of the given HCl solution = ___ N or gram.equivalent/Itr/dm3.
Amount of HCl in the given solution = _____ gms / 800 cm3.
Monday 13 September 2021
Chemistry For Class IX (New Book ) - Chapter No. 1- Fundamentals of Chemistry - Balance Equations And Numericals
GO TO INDEX
Chapter No.1- Fundamentals of Chemistry
Balance Equations And Numericals
Text Book Exercise
SECTION- D: Numerical
(1) Balance the following equations by inspection method:
(a) NH3 + O2 ⟶ NO + H2O
(b) KNO3 ⟶ KNO2 + O2
(c) Ca + H2O ⟶ Ca(OH)2 + H2
(d) NaHCO3 ⟶ Na2CO3 + H2O + CO2
(e) CO + O2 ⟶ CO2
Ans: (a) NH3 + O2 ⟶ NO + H2O
Step no.1: Write correct formula of all reactants on left side and product on right side of an equation.
NH3 + O2 ⟶ NO + H2O
Step no.2: Check the number of atoms on each side.
| Reactants | Products |
|---|---|
| N = 1 | N = 1 |
| H = 3 | H = 2 |
| O = 2 | O = 2 |
Step no.3: (Balance Hydrogen first on both side)
Now multiply the formula (NH3) with co efficient 4 on reactant side and 6 in front of water (H2O) on product side to balance the Hydrogen atoms. (if we take coefficient 2, we can not balance oxygen on both side)
4NH3 + O2 ⟶ NO + 6H2O
| Reactants | Products |
|---|---|
| N = 1 x 4 = 4 | N = 1 |
| H = 3 x 4 = 12 | H = 2 x 6 =12 |
| O = 2 | O = 1 x 6 = 6 + 1 = 7 |
Step no.4: (Balance Nitrogen first than oxygen )
Now again check and balance the equation by placing 4 in front of Nitric oxide (NO) on product side and 5 in front of oxygen on reactant side.
4NH3 + 5O2 ⟶ 4NO + 6H2O
| Reactants | Products |
|---|---|
| N = 4 | N = 1 x 4 =4 |
| H = 12 | H = 12 |
| O = 2 x 5 = 10 | O = 1 x 4 = 4 + 6 = 10 |
Now the chemical equation is balanced.
4NH3 + 5O2 ⟶ 4NO + 6H2O
(b) KNO3 ⟶ KNO2 + O2
Step no.1: Write correct formula of all reactants on left side and product on right side of an equation.
KNO3 ⟶ KNO2 + O2
Step no.2: Check the number of atoms on each side.
| Reactants | Products |
|---|---|
| K = 1 | K = 1 |
| N = 1 | N = 1 |
| O = 3 | O = 4 |
Step no.3: (Balance Oxygen on reactant side first)
Now multiply the formula of Potassium nitrate (KNO3) with co-efficient 2 on reactant side
2KNO3 ⟶ KNO2 + O2
| Reactants | Products |
|---|---|
| K = 1 x 2 = 2 | K = 1 |
| N = 1 x 2 = 2 | N = 1 |
| O = 2 x 3 = 6 | O = 4 |
Step no.4:
Now multiply the formula of Potassium nitrite (KNO2) with coefficient 2 on the product side to balance the Potassium, Nitrogen and Oxygen atoms.
2KNO3 ⟶ 2KNO2 + O2
| Reactants | Products |
|---|---|
| K = 1 x 2 = 2 | K = 1 x 2 = 2 |
| N = 1 x 2 = 2 | N = 1 x 2 = 2 |
| O = 2 x 3 = 6 | O = 2 x 2 = 4 + 2 = 6 |
Now the chemical equation is balanced.
2KNO3 ⟶ 2KNO2 + O2
(c) Ca + H2O ⟶ Ca(OH)2 + H2
Step no.1: Write correct formula of all reactants on left side and product on right side of an equation.
Ca + H2O ⟶ Ca(OH)2 + H2
Step no.2: Check the number of atoms on each side.
| Reactants | Products |
|---|---|
| Ca = 1 | Ca = 1 |
| H = 2 | H = 4 |
| O = 1 | O = 2 |
Step no.3: (Balance Oxygen first on reactant side)
Now multiply the formula (H2O) with co efficient 2 on reactant side.
Ca + 2H2O ⟶ Ca(OH)2 + H2
| Reactants | Products |
|---|---|
| Ca = 1 | Ca = 1 |
| H = 2 x 2 = 4 | H = 4 |
| O = 1 x 2 = 2 | O = 2 |
Now the chemical equation is balanced.
Ca + 2H2O ⟶ Ca(OH)2 + H2
(d) NaHCO3 ⟶ Na2CO3 + H2O + CO2
Step no.1: Write correct formula of all reactants on left side and product on right side of an equation.
NaHCO3 ⟶ Na2CO3 + H2O + CO2
Step no.2: Balance the number of atoms on each side.
| Reactants | Products |
|---|---|
| Na = 1 | Na = 2 |
| H = 1 | H = 2 |
| C = 1 | C = 2 |
| O = 3 | O = 6 |
Step no.3: (Balance Sodium first on reactant side)
Now multiply the formula of Sodium bicarbonate (NaHCO3) with co efficient 2 on reactant side.
2NaHCO3 ⟶ Na2CO3 + H2O + CO2
| Reactants | Products |
|---|---|
| Na = 1 x 2 = 2 | Na = 2 |
| H = 1 x 2 = 2 | H = 2 |
| C = 1 x 2 = 2 | C = 2 |
| O = 2 x 3 = 6 | O = 6 |
Now the chemical equation is balanced.
2NaHCO3 ⟶ Na2CO3 + H2O + CO2
(e) CO + O2 ⟶ CO2
Step no.1: Write correct formula of all reactants on left side and product on right side of an equation.
CO + O2 ⟶ CO2
Step no.2: Check the number of atoms on each side.
| Reactants | Products |
|---|---|
| C = 1 | C = 1 |
| O = 3 | O = 2 |
Step no.3: (Balance Oxygen on product side)
Now multiply the formula of Carbon dioxide (CO2) with co-efficient 2 on product side.
CO + O2 ⟶ 2CO2
| Reactants | Products |
|---|---|
| C = 1 | C = 1 x 2 = 2 |
| O = 2 | O = 2 x 2 = 4 |
Step no.4: (Balance Carbon first on the reactant side)
Now again check and balance the equation by placing 2 in front of Carbon monoxide (CO) on reactant side.
2CO + O2 ⟶ 2CO2
| Reactants | Products |
|---|---|
| C = 1 x 2 = 2 | C = 2 |
| O = 1 x 2 = 2 + 2 = 4 | O = 4 |
Now the chemical equation is balanced.
2CO + O2 ⟶ 2CO2
(2) Calculate the formula mass (a.m.u) of the following?
(i) Al2O3
(ii) MgCl2
(iii) NaCl
(iv) KNO3
i) Al2O3
Data:
Formula mass of Al2O3 =?
Solution:
Atomic mass of Al = 26.98 a.m.u
Atomic mass of O = 16 a.m.u
Formula unit = Al2O3
Formula mass of Al2O3 = 2(26.98) + 3(16)
= 53.96 + 69 + 48
= 101.96 a.m.u Ans
ii) MgCl2
Data:
Formula mass of MgCl2 =?
Solution:
Atomic mass of Mg = 24.31 a.m.u
Atomic mass of Cl = 35.5 a.m.u
Formula unit = MgCl2
Formula mass of MgCl2 = 1(24.31) + 2(35.5)
= 24.31 + 71
= 95.31 a.m.u Ans
iii) NaCl
Data:
Formula mass of NaCl =?
Solution:
Atomic mass of Na = 23 a.m.u
Atomic mass of Cl = 35.5 a.m.u
Formula unit = NaCl
Formula mass of NaCl = 1(23) + 1(35.45)
= 23 + 35.5
= 58.5 a.m.u Ans
iv) KNO3
Data:
Formula mass of KNO3 =?
Solution:
Atomic mass of K = 39.10 a.m.u
Atomic mass of N = 14 a.m.u
Atomic mass of O = 16 a.m.u
Formula unit = KNO3
Formula mass of KNO3 = 1(39.10) + 1(14) + 3(16)
= 39.10 + 14 + 48
= 101.1 a.m.u Ans
(3) Calculate the molecular mass (a.m.u) of the following?
(i) C2H5OH
(ii) H2O
(iii) NH3
(iv) CO2
(i) C2H5OH
Data:
Molecular mass (a.m.u) of C2H5OH = ?
Solution:
Atomic mass of C = 12 a.m.u
Atomic mass of H = 1 a.m.u
Atomic mass of O = 16 a.m.u
Molecular mass of C2H5OH = 2(At. Mass of C) + 6(At. Mass of H) + 1(At. Mass of O)
= 2(12) + 6(1) + 1(16)
= 24 + 6 + 16
= 46 a.m.u Ans
(ii) H2O
Data:
Molecular mass (a.m.u) of H2O =?
Solution:
Atomic mass of H = 1 a.m.u
Atomic mass of O = 16 a.m.u
Molecular mass of H2O = 2(At. Mass of O) + 1(At. Mass of O)
= 2(1) + 1(16)
= 2 + 16
= 18 a.m.u Ans
(iii) NH3
Data:
Molecular mass (a.m.u) of NH3 =?
Solution:
Atomic mass of N = 14 a.m.u
Atomic mass of H = 1 a.m.u
Molecular mass of NH3 = 1(At. Mass of N) + 3(At. Mass of H)
= 1(14) + 3(1)
= 14 + 3
= 17 a.m.u Ans
(iv) CO2
Data:
Molecular mass (a.m.u) of CO2 =?
Solution:
Atomic mass of C = 12 a.m.u
Atomic mass of O = 16 a.m.u
Molecular mass of CO2 = 1(At. Mass of C) + 2(At. Mass of O)
= 1(12) + 2(16)
= 12 + 32
= 44 a.m.u Ans
(4) How many moles are required to prepare 40 gm of H2SO4?
Data:
Given mass of H2SO4 =40g
Molecular mass of H2SO4 = 2(1) + 1(32) + 4(16) = 2 + 32 + 64 = 98 a.m.u
Number of moles =?
Solution:
By using Formula:
(5) Calculate the number of moles and number of molecules present in the following?
(a) 16 g of H2CO3
(b) 20 g of C6H12O6
(a) 16 g of H2CO3
Data:
Number of moles of H2CO3 = ?
number of molecules of H2CO3 = ?
Given mass of of H2CO3 = 16 g
Avogadro’s Number = NA = 6.02 x 1023
Solution:
Molecular mass of Carbonoc acid (H2CO3) = (2 x 1) + (1 x 12) + (3 x 16)
= 2 + 12 + 48 = 62 a.m.u
Formula:
Number of molecules = Number of moles x NA
= 0.26 x 6.02 x 1023
= 1.565 x 1023 molecules of H2CO3 Ans.
(b) 20 g of C6H12O6
Data:
Number of moles of C6H12O6 = ?
number of molecules of C6H12O6 = ?
Given mass of of C6H12O6 = 20 g
Avogadro’s Number = NA = 6.02 x 1023
Solution:
Molecular mass of glucose ( C6H12O6 ) = (6 x 12) + (12 x1) + (6 x 16)
=72 + 12 + 96 = 180 a.m.u
Formula:
Number of molecules = Number of moles x NA
= 0.111 x 6.02 x 1023
= 0.668 x 1023
= 6.68 x 1022 molecules of glucose Ans.
More Numericals
Examples From Text Book
Example 1.1:If any element have number of protons 11 and number of neutrons 12, find out its atomic number and atomic mass?
Data:
Number of protons = 11
Number of neutrons = 12
Z =?
A =?
Solution:
As we know atomic number Z is number of protons due to this
Atomic number Z = 11
Atomic mass is A = Z + n (Number of neutrons)
A = 11+12
A =23 Ans
Example 1.2:
How many number of protons and neutrons are there in an atom having A= 40 and Z= 20?
Data:
A = 40
Z = 20
Number of protons?
Number of neutrons?
Solution:
As Number of protons is Z = 20
Number of neutrons = A - Z
= 40 - 20
= 20 Ans
Example 1.3:
Calculate the molecular mass of HNO3 Solution
Data:
Molecular mass of HNO3 = ?
Solution:
Atomic mass of H = 1 a.m.u
Atomic mass of N = 14 a.m.u
Atomic mass of O = 16 a.m.u
Molecular mass = 1(At. Mass of H) +1(At. Mass of N) +3(At. Mass of O)
= 1 + 14 + 3(16)
= 1 + 14 + 48
= 63 a.m.u Ans
Example 1.4:
Calculate the Formula mass of Al2(SO4)3
Data:
Formula mass of Al2(SO4)3 = ?
Solution:
Atomic mass of Al = 26.98 a.m.u
Atomic mass of S = 32 a.m.u
Atomic mass of O = 16 a.m.u
Formula unit = Al2(SO4)3
Formula mass of Al2(SO4)3 = 2(26.98) + 3(32) +12(16)
= 53.96 + 69 + 192
= 342.14 a.m.u Ans
Example 1.5:
Calculate the number of moles in 40 g of Na.
Data:
Number of moles in 40 g of Na = ?
Solution:
Given mass of Na =40 g
Molecular m ass of Na = 23 a.m.u
Number of moles =?
Formula:
Example 1.6:
What is the mass of 4 moles of CO2?
Data:
Mass of CO2 = ?
Solution:
Number of moles of CO2 = 4 moles
Formula mass of CO2 = 44 gm
mass of CO2 = ?
Formula:
Mass of CO2 = number of moles of CO2 × formula mass of CO2
= 4 x 44
= 176 gm
Example 1.7:
Calculate the number of atoms present in 9.2 gm of Calcium (Ca).
Data:
Number of atoms of Ca = ?
Avogadro’s Number = NA = 6.02 x1023
Solution:
Atomic mass of Calcium (Ca) = 40
1 g atomic weight of Calcium = 40 gm
40 g of Calcium contains =6.02 x 1023 atoms of Calcium
Formula:
Example 1.8:
Calculate the number of moles, number of molecules present in 8 g of C6H12O6?
Data:
Number of moles of C6H12O6 = ?
number of molecules of C6H12O6 = ?
Given mass of of C6H12O6 = 8 g
Avogadro’s Number = NA = 6.02 x 1023
Solution:
Molecular mass of glucose (C6H12O6) = (6 x 12) + (12 x 1) + (6 x 16)
= 72 + 12 + 96 = 180 a.m.u
Formula:
Number of molecules = Number of moles x NA
= 0.04 x 6.02 x 1023
= 0.240 x 1023
=2.40 x 1022 molecules of glucose Ans.
Example 1.9:
A coin of silver (Ag) having 8.5 gm weight. Calculate the number of moles of silver in coin?
Data:
Given mass Of Ag = 8.5 gm
Number of moles of Ag = ?
Atomic mass of Ag = 107 a.m.u
Solution:
The mass is converted to number of moles by the following equation:
Example 1.10:
Calculate the number of moles, number of molecules and number of atoms present in 10 gm of H2SO4?
Data:
The known mass of H2SO4 = 10 gm
Molar mass of H2SO4 = 98.0 gm
Number of moles of H2SO4 = ?
Number of molecules of H2SO4 = ?
Number of atoms of H2SO4 = ?
Avogadro’s number = NA = 6.02 x 1023
Solution:
Number Of molecules Of H2SO4 = Number of moles x Avogadro’s number
= 0.10 x 6.02 x 1023
= 0.602 x 1023
= 6.02 x 1022 Ans.
Number of atoms
Number of Atoms Of H2SO4 = 2 atoms of H + 1 atoms of S + 4 atoms of O = 7
Number of Hydrogen (H) Atoms in H2SO4 = 2/7 x 6.02 x 1022
= 0.29 x 6.02 x 1022
= 1.7458 x 1022 atom of H
Number of Sulphur (S) Atoms in H2SO4 = 1/7 x 6.02 x 1022
= 0.143 x 6.02 x 1022
= 0.86 x 1022
= 8.6 x 1021 atoms of S
Number of Oxygen (O) Atoms in H2SO4 = 4 / 7 6.02 x 1022
= 0.57 x 6.02 x 1022
= 3.43 x 1022 atoms of O
Example 1.11:
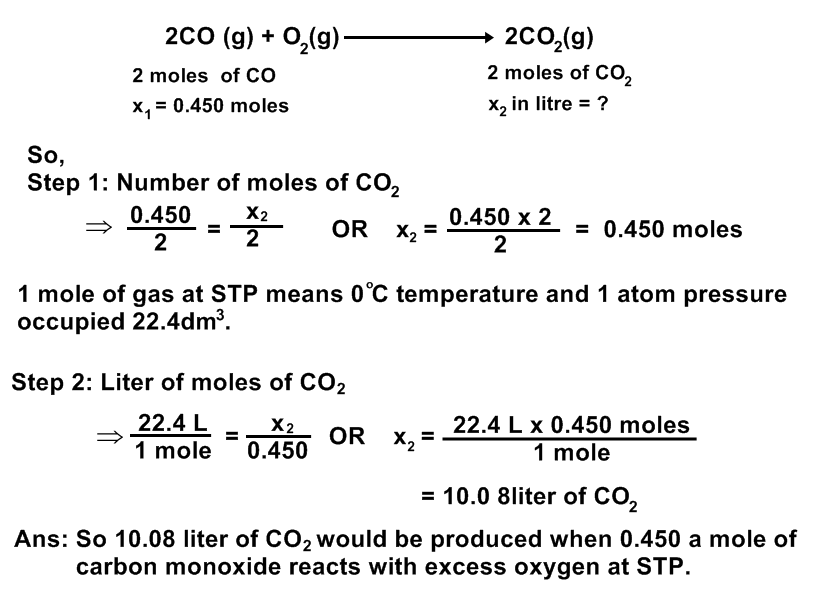
How many liters of carbon dioxide would be produced if 0.450 of a mole of carbon monoxide reacts with excess oxygen at STP.
Data:
Number of moles of carbon monoxide (CO) = x1 = 0.450 moles
Liter of carbon dioxide = x2 liters = ?
Solution:
The equation for the reaction is
Saturday 11 September 2021
Chemistry (Eng and Ur) - Past Paper 2021 (MCQs Only) - For HSC Part 2 (Home Economics Group) - For Failure, Improvements, Additional Subjects...
GO TO INDEX
Chemistry
Past Paper 2021 (MCQs Only)
For HSC Part 2 - Home Economics Group
For Failure, Improvements, Additional Subjects...
Physics - Paper II - Past Paper 2021 (MCQs Only) - For HSC Part 2 (Home Economics Group) - For Failure, Improvements, Additional Subjects...
GO TO INDEX
Physics - Paper II
Past Paper 2021 (MCQs Only)
For HSC Part 2 - Home Economics Group
For Failure, Improvements, Additional Subjects...
Chemistry For Class IX (New Book ) - Chapter No. 1- Fundamentals of Chemistry - Short Questions And Answers
GO TO INDEX
Chapter No.1 - Fundamentals of Chemistry
Short Questions And Answers
Text Book Exercise
SECTION- B: SHORT QUESTIONS:
Q.1: Differentiate between the physical and analytical chemistry?
Ans: Difference Between the Physical And Analytical Chemistry
| S.NO. | Physical Chemistry | Analytical Chemistry |
|---|---|---|
| 1. | Physical chemistry is the branch of chemistry which deals with relationship between composition and physical properties of matter with the changes in them. | Analytical chemistry is the branch of chemistry which deals with separation and analysis of kind, quality and quantity of various components in given substance. |
| 2. | It deals with the laws and principles governing the combination of atoms and molecules in chemical reactions. | It used in chromatography, electrophoresis and spectroscopy. |
Q.2: Define Molecules and Write down the classification of molecule?
Ans: Molecules:
A molecule is the smallest particle in a chemical element or Compound that has the chemical properties of that element or Compound. Molecules are made up of atoms that are held together by chemical bonds. These bonds form as a result of the Sharing or exchange of electrons among atoms.
In other words:
- Molecule is chemical combination of atoms.
- Molecule is smallest unit of a substance.
- Molecule shows properties of substance.
- Molecule can exist independently.
Classification Or Types Of Molecules:
Molecules are classifies as:
- On basis of number of atoms
- On basis of types of atoms
A) On Basis Of Number Of Atoms
1) Mono atomic Molecule:
Molecule consist of one atom is called mono atomic molecule.
e.g. Helium (He), Neon(Ne), Argon(Ar), Krypton(Kr), Xenon (Xe), Radon (Rn)
(2) Di atomic Molecule:
Molecule consist of two atoms is called di atomic molecule.
e.g. Hydrogen (H2), Oxygen (O2), Chlorine (Cl2), Nitrogen (2), Bromine (Br2), Iodine (I2)
(3) Tri atomic Molecule:
Molecule consist of three atoms is called tri atomic molecule.
e.g. H2O, CO2
(4) Poly atomic Molecule:
Molecule consist of many atoms is called poly atomic molecule.
e.g. CH4, H2SO4, C6H12O6, Ozone (O3), Phosphorus4, Sulphur8
On Basis Of Types Of Atoms:
(5) Homo atomic Molecule:
Molecule consist of same type of atoms is called homo atomic molecule.
e.g. H2, O3, P4, S8
(6) Hetero Molecule:
Molecule consist of different type of atoms is called hetero atomic molecule.
e.g. CO2, H2O, NH3
Q.3: Identify the differences among the following:
(a) Atom and Ion
(b) Molecule and Molecular ion
(c) Compound and Mixture
Ans: Difference between Atom and Ions
| S.NO. | ATOM | ION |
|---|---|---|
| 1. | Atom is the smallest particle of an element. | Ion is the smallest unit of ionic compound. |
| 2. | Atom can or can not exist independently and take part in chemical reaction. | Ion can not exist independently and surrounded by oppositely charged ions. |
| 3. | Atom is electrically neutral. | Ion has negative or positive charge. |
Difference between Molecule and Molecular ion
| S.NO. | MOLECULE | MOLECULAR ION |
|---|---|---|
| 1. | Molecule is the smallest particle in a chemical element or compound that has chemical properties of that element or compound. | Molecular ion formed by gain and lose of electrons by a molecule. |
| 2. | Molecule is always neutral. | Molecular ion have positive or negative charge. |
| 3. | Molecule is stable unit. | Molecular ion is reactive species. |
| 4. | Molecule is formed by the combination of toms. | Molecular ion formed by the ionization of a molecule. |
Difference Between Compound And Mixture
| S.NO. | COMPOUND | MIXTURE |
|---|---|---|
| 1. | Compound is formed by a chemical combination of atoms of the elements. | Mixture formed by the simple mixing of the substances. |
| 2. | Constituent of compound lose their identity and form a new substance with new properties. | Constituents of mixture retain their properties in mixture. |
| 3. | Compounds have fixed composition by mass. | Mixtures have no fixed composition by mass. |
| 4. | Components can not be separated by physical means. | The components can be separated by physical means. |
| 5. | Every compound represented by chemical formula. | It consists of two or more components and does not show any chemical formula. |
| 6. | Compounds have homogenous composition | Mixtures have homogenous as well as heterogeneous composition. |
| 7. | Compounds have sharp and fixed melting points. | Mixtures do not have sharp and fixed melting points. |
Q.4: Define the following terms:
(a) Gram atomic mass
(b) Gram molecular mass
(c) Gram formula mass
Ans: Gram Atomic Mass:
The atomic mass of an element expressed in gram is called gram atomic mass. It is also called 1 mole.
Example:
- 1 gram atom of oxygen = 16.00 g = 1 mole of oxygen atom
- 1 gram atom of carbon = 12.00 g = 1 mole of carbon atom
- 1 gram atom of nitrogen = 14.00 g = 1 mole of nitrogen atom
Gram Molecular Mass:
The molecular mass of an element or a compound expressed in gram is called gram molecular mass. It is also called 1 mole.
Example:
- 1 gram molecule of oxygen (O2) = 32.00 g = 1 mole of oxygen molecule
- 1 gram molecule of water (H20) = 18.00 g = 1 mole of water
- 1 gram molecule of ethanol C2H5OH = 46.00 g = 1 mole of ethanol
Gram Formula Mass:
The formula mass of an ionic compound expressed in grams is called gram formula mass. It is also called 1 mole.
Example:
- 1 gram formula of NaCl = 58.5 g = 1 mole of sodium chloride
- 1 gram formula mass of CaCO3 = 100 g = 1 mole of calcium carbonate
Q.5: Write down the chemical, empirical and molecular formula of the following?
Sulphuric acid, Carbon dioxide, Glucose, Benzene, Hydrogen peroxide, Acetic Acid
Ans: Compounds With Their Empirical And Molecular Formula:
| Compounds | Empirical Formula | Molecular Formula |
|---|---|---|
| Sulphuric acid | H2SO4 | H2SO4 |
| Carbon dioxide | CO2 | CO2 |
| Glucose | CH2O | C6H12O6 |
| Benzene | CH | C6H6 |
| Hydrogen peroxide | HO | H2O2 |
| Acetic Acid | CH2O | CH3COOH |
Q.6: What is Free Radical?
Ans: FREE RADICALS:
Free radicals are atoms and group of atoms having number of unpaired electrons. It is represented by putting a dot over the symbol of an element.
For example: H° , Cl° ,H3°, C°
Formation Of Free Radicals:
Free radicals are formed when homolytic breakage of bond between two atoms takes place by the absorption of heat or light energy. Free radical is very reactive chemical species.
Q.7: Describe relationship between empirical and molecular formula? Explain with examples.
Ans: Relationship Between Empirical and Molecular formula:
The empirical formula of a compound gives the simplest ratio of the number of different atoms present, whereas the molecular formula gives the actual number of each different atom present in a molecule. If the formula is simplified then it is an empirical formula. The molecular formula is commonly used and is an integral multiple (1,2,3 etc.) of the empirical formula.
The general statement relating molecular formula and the empirical formula is:
Molecular Formula = (Empirical Formula)n
OR
Molecular Formula = n x Empirical Formula
where n=1,2,3,etcExample No.1: Benzene has molecular formula C6H6. Which has simplest ratio of hydrogen and carbon as follows:
C6H6
6:6
1:1
CH
So the empirical formula of benzene is CH and have simple ratio is 1:1 of atoms in molecule of benzene.Example No.2: Glucose has molecular formula C6H12O6 . It shows the ratio as follows:
C6H12O6
6:12:6
1: 2: 1
C:H2:O
So the empirical formula of glucose is CHO and have simple ratio 1:2:1 of atoms in molecule of glucose.Q.8: Explain why hydrogen and oxygen are considered as element whereas water is compound?
Ans: Reason:
Hydrogen and oxygen are made up of same kind of atom hence these are considered as elements whereas water is made by the combination of different kinds of atoms such as hydrogen and oxygen, hence it is called as a compound and not an element.
BASIC DEFINITIONS
Q.9: Define:- Science
- Chemistry
- Matter , States Of Matters and Classification Of Matter
- Atom
- Substance
- Valency
- Compounds
Ans: 1. SCIENCE:
Word science comes from latin word “Scientia” which means “ knowledge, This knowledge is based on hypothesis observation and experiments of universal science.
2. CHEMISTRY:
Chemistry is the branch of science which deals with the properties, composition and structure of matter. Chemistry also deals with the changes involved in the matter.
3. MATTER:
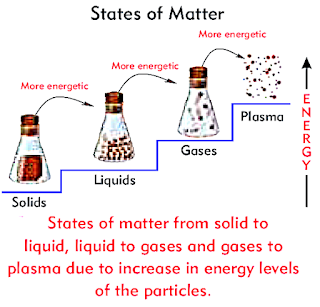
Matter is all around us. Matter is simply defined as anything that has mass and occupies space.
States of Matter:
It is found in three common states:
- Solid
- Liquid
- Gas
- The plasma is also considered as fourth state of matter.
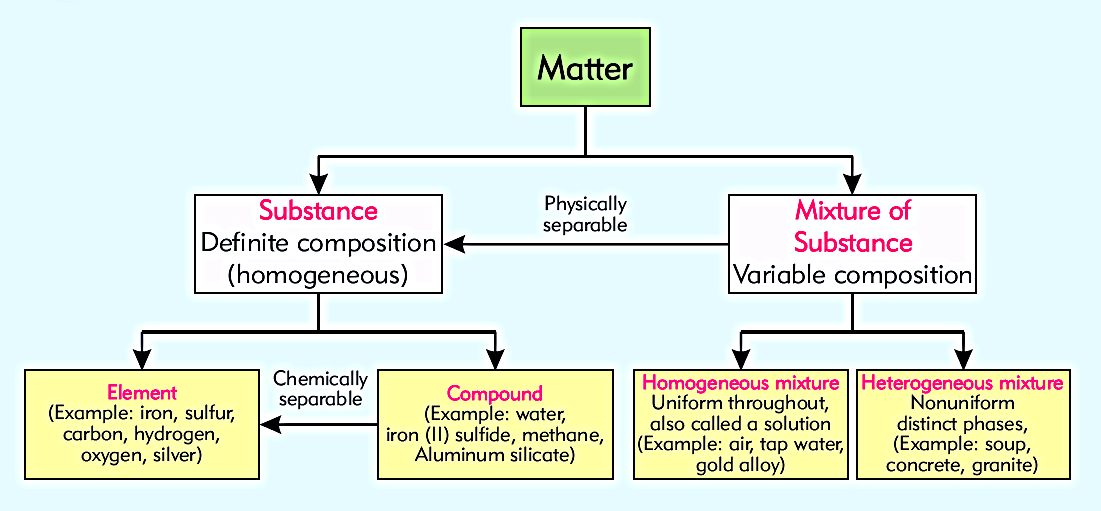
Classification Of Matter:
4. ATOM:
- Matter is made up of smallest particles which are known as atom.
- Atoms are basic units of matter and definite structure of elements.
- Atoms are made up of three particles: proton, neutrons and electrons. Which are composed of even small particles.
- Neutron and proton are situated in nucleus and electrons are revolving around the nucleus.
5. SUBSTANCE:
- A piece of matter in pure form is termed as a substance.
- Every substance has a fixed or definite composition and specific properties.
- They are homogeneous in nature.
- Substances are elements and compounds.
-
Example:
Element: Tin, iron, sulphur, diamond or carbon, hydrogen, oxygen, silver)
Compound: water, iron (II) sulfide, methane, Aluminum silicate. pure sugar (sucrose). table salt (sodium chloride). They are Chemically separable into their elements.
6. VALENCY:
- The Combining power of an element with other element is called valency.
- The valency depends upon the number of electrons in the outermost shell.
- Valency is the number of electron of an atom of an element can gain, lose or share.
-
Example:
(i) Hydrogen (H) has +1 valency.
(ii) Oxygen (O ) has -2 valency.
(iii) Aluminium (Al) has +3 valency.
(iv) Carbon (C) has +4 and +2 valency etc.
7. Compounds:
The Compound is a substance formed when two or more elements are chemically bonded together in a fixed ratio by mass, As a result a new entirely different properties possessing substance formed.
The type of bonds holding elements may be:
- ionic bonds or
- covalent bonds
-
Ionic compounds:
(i) Sodium Chloride (NaCl)
(ii) Copper sulphate (CuSO4)
(iii) Potassium bromide (KBr) etc. - Covalent compounds:
(i) Water (H2O)
(ii) Methane (CH4)
(iii) Sulphuric acid (H4SO4) etc.
Q.10: Define element and its types?
Ans: Element:
- An Element is a substance made up of same type of atoms.
- Having same atomic number.
- It cannot be decomposed into Simple substances by ordinary chemical reaction.
- Elements occur in nature in free or combined form in solid, Liquid and gases states.
- Now 118 elements have been discovered.
- Example: Majority of elements are solids as copper, gold, zinc etc. Very few elements are liquid as mercury and bromine. Few elements are gases as hydrogen, oxygen and nitrogen.
Types Of Elements:
Elements are divided into metals, nonmetals and metalloids on the basis of their properties.
Metal:
- Metals are solid material.
- They are typically hard, shiny, malleable, fusible, and ductile.
- They have good electrical and thermal conductivity.
- e.g. iron, gold, silver, and aluminium, and alloys such as steel.
Non metals:
- Non metal is an element that doesn't have the characteristics of metal.
- They do not have ability to conduct heat or electricity.
- They do not have luster, or flexibility.
- e.g. An example of a nonmetal element is carbon.
Metalloids:
- Metalloid is an element whose properties are intermediate between those of metals and solid non-metals.
- They are semi conductors.
- e.g. arsenic, antimony, or tin.
Q.11: What are symbols Or How to write Symbol?
Ans: Symbol:
Symbol is an abbreviation to represent the name of elements. A symbol is taken from the name that elements from English , Latin, Greek and German.
- Symbols are usually one or two letter long.
- Every symbol starts with capital letter
E.g. as carbon with C or sulphur as S. - If symbol has second letter then start with capital and second will be in small letter
E.g. as He for helium, Na for sodium, Cr for chromium.
Q.12: What is Chemical formula?
Ans: CHEMICAL FORMULA:
The compounds are represented by Chemical Formula as elements are represented by symbols with respect to valencies.
OR
The chemical formula represent the symbol of elements and ratios of elements to one another in a compound.OR
Chemical formula tells us number of atoms of each element in a compound with symbols.For Example:
- Chemical formula of water is H2O which indicates that 2 atoms of hydrogen combines with 1 atom of oxygen.
- Chemical formula of ammonia NH3 shows that one nitrogen atom combines with 3 atoms of hydrogen.
Q.13: Define mixture and its types?
Ans: MIXTURE:
When two or more elements or compounds physically combines without any fixed ratio is known as Mixture. The component substances retain their chemical properties. Mixtures can be separated again by physical methods, such as Filtration, Evaporation, Distillation and Crystallization. Mixture has variable composition.
Types of Mixture
There are two main types of mixtures, which are:
- Homogeneous mixture and
- Heterogeneous mixture.
Homogeneous mixture:
In a homogenous mixture all the substances are evenly distributed throughout the mixture, or It is uniform throughout, also called a solution.
Example: air, tap water, gold alloy, Salt water, blood etc.
Heterogeneous mixture:
In a heterogeneous mixture the substances are not evenly distributed or It has nonuniform distinct phases.
Example: chocolate chip cookies, pizza, rocks, soup, concrete, granite etc.
Test Yourself
Q.1: In which branch of chemistry analysis of quality and quantity of compounds studied:Ans: Analytical Chemistry:
Analytical chemistry is the branch of chemistry which deals with separation and analysis of kind, quality and quantity of various components in given substance. It used in chromatography, electrophoresis and spectroscopy.
Q.2: What happens due to deficiency of bimolecules?
Ans: Deficiency or disorder of bimolecules cause diseases in living organisms (plants and animals).
Q.3: List some uses of salts in society?
Ans: Uses Of Different Salts:
1. Sodium Chloride (NaCl) is used in food flavouring. It is also used in the preparation of different chemicals in chemical industries.
2. Sodium nitrite (NaNO2), Sodium sulphite (Na2SO3) and Sodium citrate (Na3C6H5O7) use as Food preservatives.
3. Silver Salts use in photography.
4. Ammonium sulphate [(NH4)2SO4], Ammonium nitrate (NH4NO3) are used as Fertilizers in agriculture.
5. Plaster of Paris” (Calcium sulphate CaSO4) uses in medical.
Q.4: Identify and list down examples of green chemistry in our environment?
Ans: Pesticides, Safer chemical (polyphenylsulfon), less hazardous chemical (poly carbons) and safer solvents are examples of green chemistry in our environment.
Subscribe to:
Posts (Atom)