Q.1: Define energy?
Ans:
ENERGY:
Energy is the capacity for doing work. The only source of energy on earth is Sun. Energy can neither be created nor be destroyed but it can change from one form to another form. It may exist in potential, kinetic, thermal, electrical, chemical, nuclear, or other various forms.
Q.2: From where does the energy come in fuel and food molecules?
Ans:
Source Of Energy:
The only source of energy on earth is Sun. Energy of the Sun reaches earth in the form of light (light energy). This light energy is converted into chemical energy by living organisms or in heat energy stored by non-living things.
Conversion Of Energy:
Law of conservation of energy or first law of thermodynamics states that:
"energy can neither be created nor be destroyed but it can change from one form to another form.
Thus the heat energy of light converts in:
- K.E. energy which flows water. This K.E. of water in dams is converted into mechanical energy when falls on turbine. This mechanical energy converts into light energy in bulbs and LED lights or again in mechanical energy in our fans.
- Light energy when falls on green parts of plant is captured, It is:
(a) Converted into chemical energy. This chemical energy is stored as food energy in plants. When these parts of plant are eaten by animal this energy transferred into them.
(b) When the organisms buried and remain under pressure inside earth crust for millions of year their chemical energy is converted into fossil fuel.
Q.3: Define bioenergetics?
Ans:
BIOENERGETICS:"The study about conversion of free energy into different forms by living organisms is called Bioenergetics."
Bioenergetics can also be defined as:
"The study of energy relationships, energy transformation and transmission in living organisms."
It is the part of biology, Physics and chemistry concerned with the energy involved in making and breaking of chemical bonds found in the molecules of organisms.
Q.4: Describe the chemical process of energy transmission in living organisms?
Ans:
CHEMICAL PROCESS OF ENERGY TRANSMISSION:
In living organisms the energy is transferred through gain or loss of electrons during formation and breaking of chemical bonds. There are two chemical processes where it occurs, known with the name of oxidation and reduction.
The Oxidation Reactions:
are those reactions in which loss of electron (e
-) and proton (H
+) occurs. These electrons carry energy from the molecules from where they release to the molecules where they added. e.g.
Iron reacts with oxygen to form a chemical called rust, in this reaction iron (Fe) loses some e- which transfer to oxygen. In this reaction Fe is oxidized and it transfers its energy to oxygen through electrons.
The Reduction Reactions:
are those reaction in which gain of e
- and H
+ occur. This gain of e
- also brings energy which is stored in it.
Redox Reaction:
In living organisms these oxidation - reduction (Redox) reactions occur continuously to transfer energy from one molecule to other molecule, without these reactions energy transfer becomes impossible in living system.
Q.5: What is energy currency of cell? OR Describe the formation of ATP?
Ans:
Energy Currency in living organism:
The major energy currency of all cells of living organism is a nucleotide called adenosine triphosphate (ATP) because it acts as the main energy source for majority of the cellular functions.
Formation Of Adenosine Tri-Phosphate (ATP):
As all living organisms have system to store energy. This energy is stored in a special molecule called Adenosine Tri-Phosphate (ATP). In organisms, energy is liberated during any oxidation reaction; this energy is utilized by molecules called Adenosine Di-Phosphate (ADP) to form a bond with phosphate (P). As a result the ADP become ATP, energy of oxidation is now stored in ATP.
Use Of Energy Stored In ATP:
The amount of energy stored is 7.3 Kcal / mole, this stored energy in ATP will be utilized by living organism for performing any type of work e.g. transport of molecules against the concentration gradient. The energy is now become free (liberated) by breaking ATP molecule.
ATP → ADP + P + Energy (7.3 K Cal / mole)
So the formation of ATP is endergonic (energy intake) process and breakdown of ATP is exergonic (energy liberating) process.
Q.6: Define and describe the process of photosynthesis and write down its importance?
Ans:
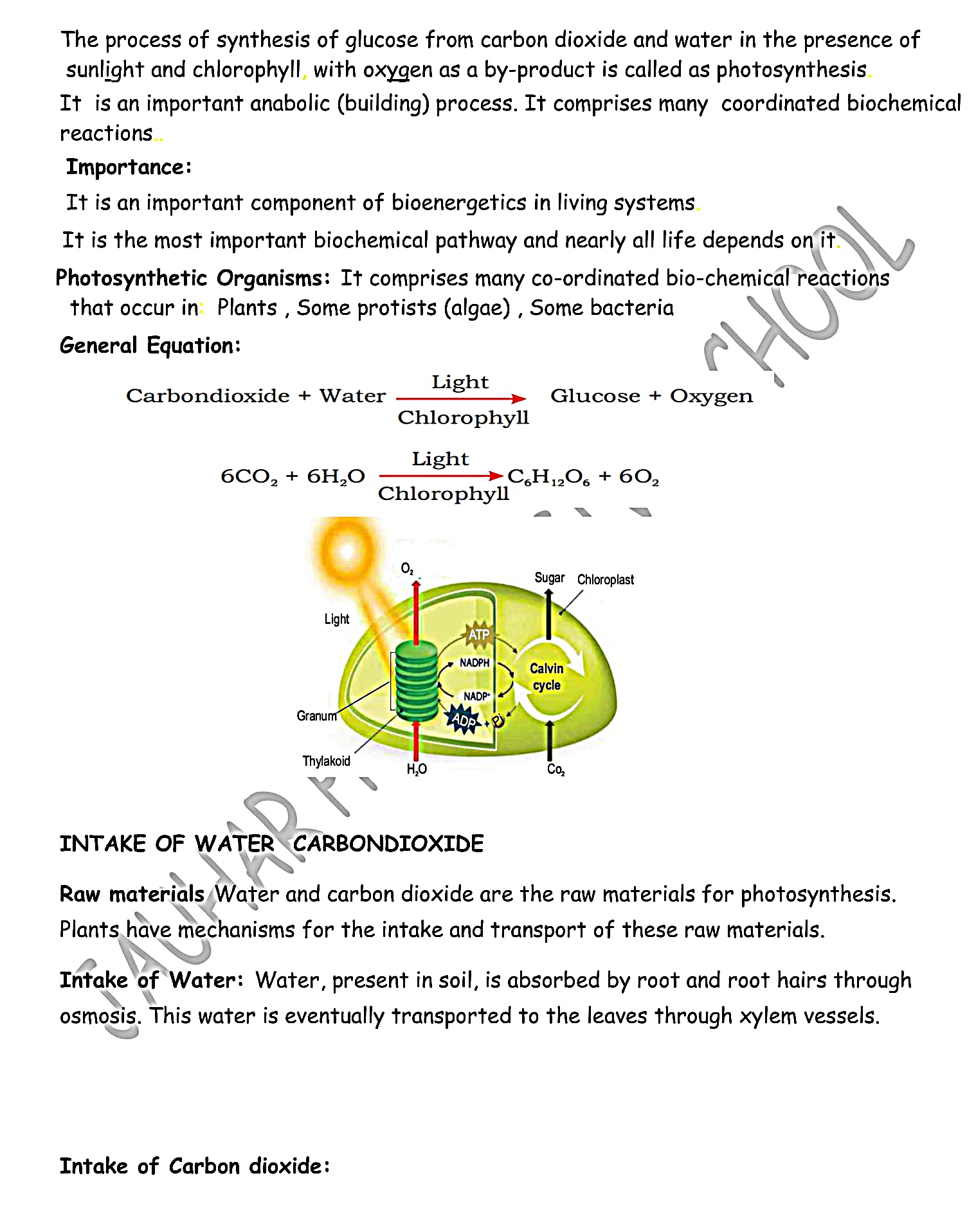
PHOTOSYNTHESIS:
Word photo means light and synthesis means to prepare.
Photosynthesis is the fundamental process in which basic organic molecules and O
2 are produced for all bio-molecules and living organisms. Photosynthesis converts light energy into chemical energy.
Definition:
Plants utilize simple inorganic molecules carbon dioxide (CO
2) and water (H
2O) which react by using light energy in the presence of pigments like Chlorophyll to form glucose and oxygen.
OR
Photosynthesis is a biochemical process by which green part of plant manufactures their own food (Glucose C
6H
12O
12) by combining carbon dioxide and water in the presence of sunlight and chlorophyll. During this process oxygen is liberated.
Photosynthetic Organism:
This process is carried out by chlorophyll containing organisms like plants, algae, some protozoan and some bacteria.
Equation:
IMPORTANCE OR SIGNIFICANCE OF PHTOSYNTHESIS:
(i) Role Of Glucose:
The fundamental molecule produced during photosynthesis is simple sugar i.e. Glucose. Glucose utilizes in most of the metabolism of plant to produce secondary products like starch and other polysaccharides. Plants also use carbohydrates to form fats, proteins and other chemical like Nucleic Acids. This glucose is also used in respiration as reactant to produce energy for the metabolism of living organisms.
(ii) Essential In Food Chain:
Plants are not the only organisms which depend on photosynthesis but animals (Heterotrophs) also depend on phototrophs (i.e plants). Animals utilize the molecules of phototrophs as food molecules. If an animal is herbivorous it feeds directly on plants. If an animal is carnivorous it depends on herbivores (those animals which feed on plants). These feeding sequences and relationship are called Food Chains.
(iii) Role Of Oxygen:
Photosynthesis is the only process which produces free O
2 by splitting water. This O
2 is utilized by all living organisms for respiration to produced energy for metabolism. Without O
2 living organisms cannot survive.
(iv) Quantity Of CO2 and O2 In nature Is Maintained:
During Photosynthesis plants fix CO
2 and release O
2 in environment. CO
2 has a property to absorb heat of sun. If its quantity increases in environment, there will be increase in an environmental temperature on earth called Global warming. Thus photosynthesis keeps the quantity of CO
2 to maintain the temperature of earth.
Q.7: What is chloroplast and chlorophyll? OR Define chloroplast and chlorophyll?
Ans:
CHLOROPLAST:
Green part of plants and algae contain special cell which contain special organelle called chloroplast. The chloroplast is a double membraned organelle. Within the double membrane is a gel-like substance (semi fluid protein) called stroma. Stroma contains enzymes for photosynthesis. Suspended in the stroma are stack-like structures called grana (singular = granum). Each granum is a stack of thylakoid discs.
Chlorophyll:
The chlorophyll molecules are green pigments, found on the surface of the thylakoid discs in chloroplast. It captures a specific part of visible light only from sun, therefore it is not a reactant but absorbs energy needed to drive the photosynthetic reaction.
Q.8: Describe the Process Of Photosynthesis? Or How many phases of photosynthesis are there describe each with suitable diagram?
Ans:
PROCESS OF PHOTOSYNTHESIS:
The simple looking reaction of photosynthesis is not as simple. It involves number of chemical reactions which are catalyzed by number of enzymes, either in non cyclic or cyclic ways. Each reaction occurs at different site in chloroplast.
PHASES OF PHOTOSYNTHESIS:
Processes of Photosynthesis is mainly divided into phases or reactions.
- Light Reaction or Light Dependent reaction
- Dark Reaction or Light Independent reaction
1. Light Reaction or Light Dependent Reaction:
Definition:
Reaction in which light energy converted into chemical energy and stored in ATP (Adenosine Triphosphate) and NADPH
2 (reduced Nicotine amide Adenosine Dinucleotide Phosphate). This conversion occurs at thylakoid membrane where solar energy is captured by pigments located in harvesting complex. This phase of photosynthesis is called
Light Dependent reaction Or Light Reaction.
It is non Cyclic process coupled with breakdown of H
2O molecules i.e. photolysis, takes place also at thylakoid membrane.
Explanation:
The term light reaction or light dependent reaction is used due to the reason that during this phase of photosynthesis light energy is captured and converted into chemical energy.
Photolysis:
Some of the light is utilized to split water into oxygen and H with e
- (electrons), this splitting of water is called Photolysis. Oxygen which is produced during photolysis is released in the environment where as H
+ together with CO
2 are used in building Glucose.
Photosystem I & II:
In chloroplast, different pigments absorb light of different wave lengths. Among them chlorophyll is the main light capturing molecules in thylakoid membrane which absorbs violet, blue and red light but reflects green therefore it appears green. In the thylakoid membrane other pigments and electron carrier molecules form highly organized assemblies in a series called photosystems. Each thylakoid contains thousands of copies of two different kind of photosystems called photosystems I and II. Each consists of two major parts:
-
A light harvesting complex and
-
An electron transport system.
The conversion of light energy takes places when the chlorophyll of reaction center receives energy. One of the electrons from chlorophyll “a” molecule leaves and jump over the electron transport system. This energized electron moves from one carrier to next. The electron releases energy, when it comes down, this energy drives reactions and produces two energy rich compounds. These are:
- ATP (Adenosine Triphosphate)
- NADPH2 (Reduced Nicotinamide Adenosine Dinucleotide Phosphate)
Phosphorylation:ADP is the compound which is already present in cell. It combines with phosphate by using energy of photon released from when moving through e- carriers in photosynthesis. The formation of ATP from ADP by using light energy called phosphorylation
Reduction Of NADP:
NADP also present in chloroplast is reduced into NADPH
2 by accepting Hydrogen ions (H
+, released from splitting of water.
ATP and NADPH
2 formed, both are energy rich compounds which are utilized in dark reaction.
2. Dark Reaction or Light Independent reaction:
Definition:
Reaction in which captured solar energy transferred to glucose from ATP and NADPH
2. It takes place in stroma, in cyclic manner. This phase of photosynthesis is called
Light Independent reaction Or Dark Reaction.
During this phase fixation of atmospheric CO
2 also takes place to form organic molecules.
Explanation:
This phase of photosynthesis does not require energy of photon but it also takes place in day simultaneously with the light reaction.
Calvin-Benson Cycle:
The ATP and NADPH
2 synthesized during the light dependent reaction are dissolved in stroma where, they provide energy to power the synthesis of Glucose from CO
2 and H
2O (i.e. H
+ and e
- of water).
This Phase occurs independently, without light as long as ATP and NADPH
2 are available. This phase of photosynthesis is cyclic phase. This phase occurs in set of reactions also called Calvin – Benson Cycle due to it's discoverer or the C
3 (three Carbon Containing Compounds formed initially) Cycle.
The C
3 Cycle requires:
- CO2 - normally from air some of it also comes from respiration.
- CO2 Capturing Sugar - a Pentose Sugar.
- Enzymes to catalyze all the reactions.
- Energy from ATP and NADPH2 come from light dependent reaction.
Q.9: Define limiting factors? Describe the limiting factors of photosynthesis?
Ans:
Limiting Factor:
Rate of biochemical reaction dependent on some factors which affect the rate are called limiting factor.
For example: At low light intensity rate of photosynthesis increase continuously but at high light intensity the rate becomes constant.
Limiting Factors For The Rate Of Photosynthesis: are,
-
Light intensity,
-
Carbon dioxide concentration and
-
Temperature
Following graph shows the idea of limiting factor.
-
A- At point Z on graph, light intensity is limiting factor.
-
B- If light intensity increase to bright light and moderate temperature the concentration of CO2, in air becomes limiting factor.
-
It is clearly observed that the same plant if put into air containing high CO2 then the rate of photosynthesis becomes high.
-
If there is high light intensity and high CO2 concentration then the temperature becomes the limiting factor but the temperature should not be very high otherwise enzymes become denatured.
Q.10: Find out the effect of light intensity on the rate of photosynthesis by experiment?
Ans:
Experiment To Show The Effect Of Light Intensity On The Rate Of Photosynthesis:
Apparatus:-
Large beaker of water,
-
Boiling tube,
-
Stand and clamp,
-
Paper clip,
-
Fresh water plant hydrilla,
-
Ruler,
-
Stopwatch,
-
Thermometer,
-
Lamp etc.
Procedure:- Take a healthy piece of Hydrilla. Place it upside down in a boiling tube of water. It helps to sink the Hydrilla.
- Clamp the tube to hold it upright in beaker of water. Ensure that the plant is perpendicular to source of light. The beaker of water is needed
to maintain a constant temperature.
- Place a thermometer in water to record the temperature. Turn off the room lights to reduce back ground light and place a bench lamp close
to the beaker.
- Observe the plant for few minutes, you will see the bubbles of gas coming out from the cut end of plant. If no bubbles are seen repeat the
experiment by using fresh piece of plant. Count the number of bubbles per minute. If the rate of bubbling is too fast to count, move the lamp
away from the breaker until the rate becomes countable.
- Repeat the counts until you are sure that the rate is constant. Record the rate and the distance of the lamp from the plant.
- Change the distance of lamp from plant and take more measurement of the rate of bubbling at each distance. Take 3 values at each point.
- Repeat the counts at different distance from plant keep the temperature of water constant thought out the experiment.
Conclusion:Suppose that the rate of bubble production is the measure of the rate of photosynthesis.
It is concluded that the rate of photosynthesis decreases at low light intensities. As the lamp is moved away from plant, the intensity of light falling on it also decreases.
Q.11: Define respiration?
Ans:
RESPIRATION:
To carry out all the life process cells requires energy. The source of this energy is food or photo assimilates (Products of photosynthesis) in plants.
Definition:"Cells break food molecules to release their Chemical energy. The breakdown of food molecules to release energy is called respiration."
Usually cells use oxygen to oxide food. It results CO
2 and water as waste products. The main food oxidized is the sugar i.e. Glucose.
Equation:
The overall equation for this chemical reaction is:
Glucose + Oxygen → Carbon dioxide + water + Energy
C6H12O6 + 6O2 → 6CO2 + 6H2O + ATP
Above equation shows that one molecule of glucose reacts with six molecules of oxygen to produce six molecules of carbon dioxide and six molecules of water. The main product is energy which is produced in the form of energy rich molecules called ATP.
Q.12: Write down the difference between breathing and respiration or cellular respiration?
Ans:
DIFFERENCE BETWEEN BREATHING AND RESPIRATION OR CELLULAR RESPIRATION:
| S.No |
Breathing |
Cellular Respiration |
|---|
| 1. | Breathing is a physical process in which O2 is taken in CO2 is given out. | Respiration is a biochemical process in which food is oxidized into CO2 and H2O. |
| 2. | It occurs outside the cells. | It occurs inside the cells. |
| 3. | There is gradual and stepwise release of energy. | There is no release of energy. |
| 4. | Enzymes are not involved. | Enzymes are involved. |
| 5. | Oxygen is necessary in this process | Oxygen is not necessary in anaerobic respiration. |
Q.13: What are the steps of respiration?
Ans:
Steps Of Respiration:
Respiration consist of the following two steps:
-
Breathing
-
Cellular Respiration
Breathing:
Breathing allows the process of gaseous exchange at surface of cells and tissues. It is the movement of air in and out of the organism to supply O
2 and give out CO
2. Breathing is also called Ventilation.
Cellular Respiration:
It is the biochemical reaction in which the oxidation of food takes place within cells with the help of oxygen and enzymes, resulting in the release of energy.
Q.14: How many types of respiration are there? Explain them with the help of chemical equations?
Ans
: TYPES OF RESPIRATION:
There are two types of respiration found in living organisms for the production of energy.
- Anaerobic Respiration or Fermentation
- Aerobic Respiration
(i) ANAEROBIC RESPIRATION OR FERMENTATION:
Definition:
The primitive type of respiration which takes place in the absence of O
2 or without O
2 is called anaerobic respiration or fermentation.
There are special conditions where O
2 is not available so the organisms adapt themselves to break down their food without oxygen which is called anaerobic respiration or fermentation.
Example:
It takes place in some bacteria, fungi, endoparasite and sometimes in animal.
MECHANISM OF ANAEROBIC RESPIRATION OR FERMENTATION:
During anaerobic respiration, glucose is not broken down completely so less amount of energy (5 to 10% as compared to aerobic respiration) is released but it can sustain life even in the absence of O
2.
It had evolved on earth where there was no O
2 on earth.
Types Of Anaerobic Respiration OR Fermentation
There are two types of anaerobic respiration:
-
Alcoholic Fermentation
-
Acidic fermentation
Alcoholic Fermentation:
The bacteria and fungi respire aerobically but when these organisms are deprived of oxygen they stop respiration aerobically and start respiring anaerobically instead, During this anaerobic respiration they produce ethyl alcohol with CO
2.
Glucose → Ethanol + CO2 + Some energy
C6H12O6 → 2C2H5OH + 2CO2 + Some ATP
Acidic Fermentation:
In animals when aerobic respiration is not enough to produced required energy they start anaerobic respiration. During this process glucose breaks down into a substance called lactic acid.
Glucose → Lactic acid + Some energy
C6H12O6 → 2C3H6O3
A limited amount of energy is produced as compared to aerobic respiration which is enough to power the athlete's muscles during muscles fatigue.
(ii) AEROBIC RESPIRATION:
Definition:Type of respiration where food breakdown occurs in the presence of oxygen to produce energy, is called aerobic respiration.
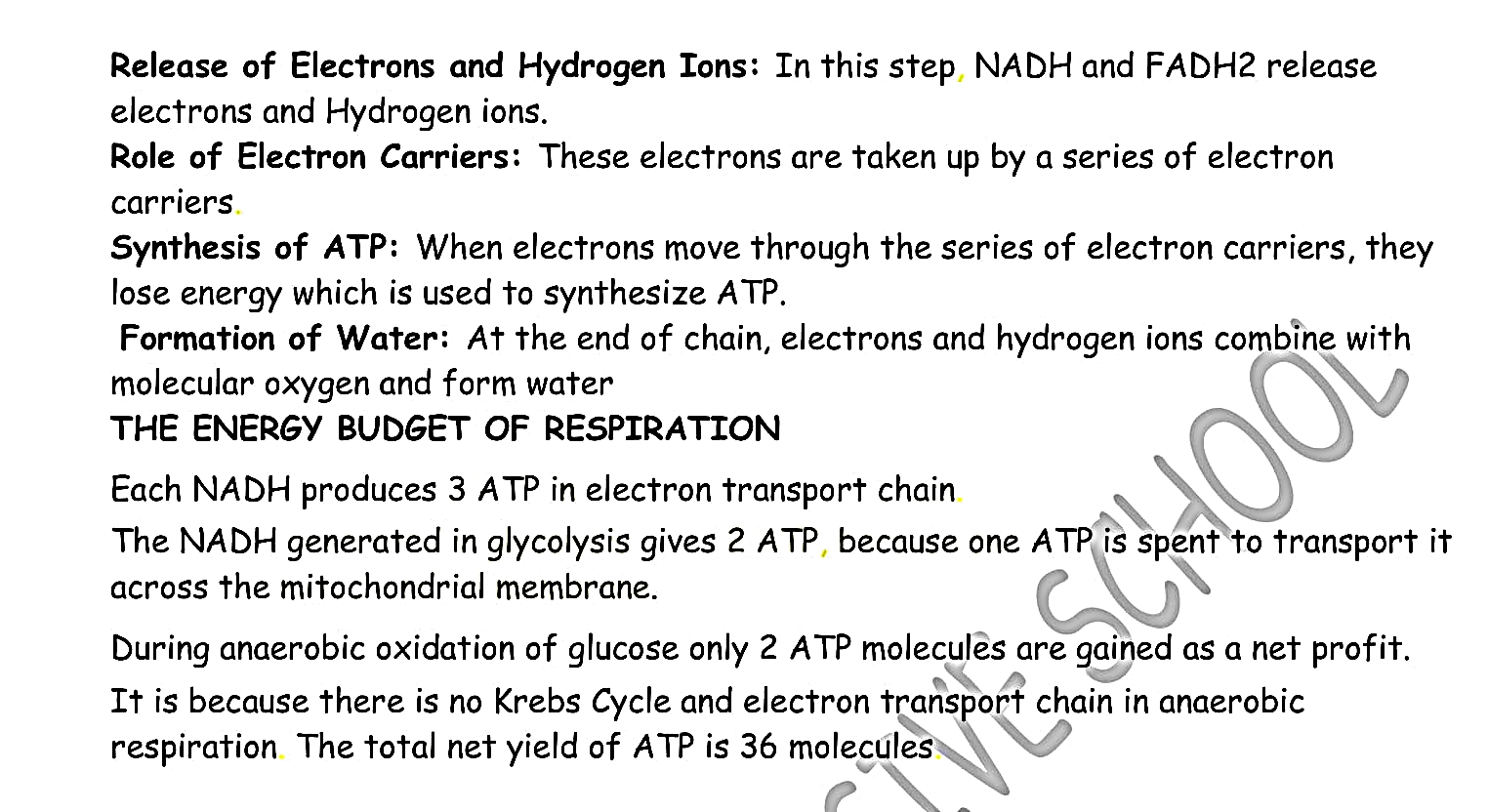
It takes place in the presence of free oxygen, oxidizing the food and releasing the maximum amount of energy i.e. 2827 kj / mole of glucose or 36 ATP molecules/glucose.
Example:
It is a method of respiration found in majority of organisms.
Equation:
The end products of aerobic respiration are CO
2 and H
2O.
Glucose + oxygen → Carbon dioxide + water + Energy (36 ATP)
C6H12O6 + 6O2 → 6CO2 + 6H2O + 36 ATP
MECHANISM OF AEROBIC RESPIRATION:
Aerobic Respiration takes place in 3 steps at different places in the cell.
-
Glycolysis
-
Kreb's or Citric acid Cycle
-
Electron Transport Chain
(a) Glycolysis (Gr. Glyco = Sugar, Lysis = Break down):
First stage is that stage where a molecule of glucose (Six carbon sugar) is broken down into two molecules of pyruvic acid (three carbon acid). It does not require oxygen. It takes place in both aerobic and anaerobic respiration. This splitting of glucose releases small amount of energy of glucose which is enough to generate 2 molecules of ATP. Glycolysis is a complex sequence of reaction all occur in cytosol.
(b) Kreb's or Citric acid Cycle:
The second stage of aerobic respiration in which pyruvic acid produced during glycolysis enters the mitochondria where O
2 available. Cellular respiration uses this O
2 to break pyruvic acid completely into CO
2 and H
2O in a cyclic manner. During Kreb's Cycle some ATP produce and some coenzymes like NAD and FAD are reduced to NADH
2 and FADH
2 . It takes place in matrix of mitochondria.
(c) Electron Transport Chain:
The last stage of aerobic respiration in which NADH
2 (Nicotinamide Adenosine Di-nucleotide) and FADH
2 (Flavinamide Adenosine Di- nucleotide) are oxidized to produce ATP and H
2O. It takes place at the cristae of mitochondria.
Q.15: Write down importance of anaerobic respiration?
Ans:
Importance Of Anaerobic Respiration:-
Anaerobic respiration is the emergency arrangement of energy which has an advantage that organisms can survive without O2 or can work with same pace for short period.
-
The other products of anaerobic respiration are acids. Vinegars are also organic acids that are produced commercially by acidic formulations.
-
Anaerobic respiration also produces ethyl alcohol. This process is commercially utilized by making alcoholic products like beer, wines and other beverages.
-
Baking industry is also based on it because anaerobic respiration also produces CO2 which gives fluffy and soft shapes to cakes and breads also break down of starch into complex sugar to form bread and pizza.
Q.16: Describe usage of respiration Energy in the body of organisms?
Ans:
Usage Of Respiration Energy In The Body Of Organisms:
Number of Processes requires energy in the body of an organism. Body provides it from respiratory energy. Following are some process which utilize respiratory energy:
-
Synthesis of molecules:
Formation of different molecules as well as large molecules from small molecules requires energy. -
Cell division:
During cell division formation of large molecules like DNA and protein takes place which require energy as well as movement of chromosome also require energy. -
Growth without cell division:
Enlargement & growth is not possible and both require formation of molecules which require energy. -
Active transport:
Movement of ions and molecules from low concentration to high concentration requires energy. -
Muscle Contraction:
Movement of muscle requires energy which is produced from chemical energy, chemical energy converted into kinetic energy. -
Passage of Nerve impulse:
Nerve Impulse (message of Neuron) is basically electrical signals moving long nerve fiber by active transport requires energy. -
Maintenance of Body temperature:
In higher animal's body temperature is maintained at constant level, this temperature maintenance requires energy of respiration.
Q.17: Distinguish between:
Photosynthesis and Respiration
Light and dark reaction
Aerobic and anaerobic respirationAns:
(i) Difference Between Photosynthesis & Respiration
| S.No. |
Photosynthesis |
Respiration |
|---|
| 1. | Photosynthesis is the process where light energy converted in chemical energy. | Respiration is the process where chemical energy converted into energy of ATP. |
| 2. | It occurs only in chlorophyll containing organisms.
OR It takes place in green part i.e. chlorophyll of the cells of plants only. | It occurs in all organisms.
OR It takes place in all living cells of plants and animals. |
| 3. | It requires light sources i.e occur only in the presence of light. (i.e. during day time only) | It does not requires light source so occurs throughout the life. (i.e. during day and night like.) |
| 4. | It occurs in chloroplast. | It occurs in mitochondria. |
| 5. | The reactants are Carbon dioxide and water. | Reactants are carbohydrates and oxygen usually. |
| 6. | The end products are glucose (simple carbohydrate) and oxygen. | The end products are carbon dioxide and water in the case of aerobic respiration |
| 7. | It is an anabolic process i.e. compounds are formed. | It is catabolic process i.e. compounds are broken down. |
| 8. | Energy is used in this process ans it is stored in the form of food material. | In respiration energy is released from food materials. |
| 9. | During this process carbon dioxide enters in the plant body and oxygen is given out. | During this process oxygen is entered in the body and carbon dioxide releases. |
| 10. | The chemical equation for this process is:
6CO2 + 6H2O → C6H12O6 + 6O2
| The chemical equation for this process is:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
|
| 11. | During the breakdown of one glucose, 38 ATP molecules are formed. | During the formation of one glucose, 18 ATP molecules are utilized. |
Difference Between Light Reaction And Dark Reaction
| S.No. |
Light Reaction |
Dark Reaction |
|---|
| 1. | It take place only in the presences of light | It takes place only in presence or absence of sunlight. |
| 2. | It is a photo-chemical phase. | It is a biochemical phase. |
| 3. | It takes place in the grana of the chloroplast | It takes place in the stroma of the chloroplast. |
| 4. | NADP utilizes H+ ions to form NADPH. | The hydrogen of NADPH combines with CO2. |
| 5. | The end products are ATP and NADPH2. | Glucose is the end product. ATP and NADPH2 help in the formation of glucose. |
| 6. | The water molecules split into hydrogen and oxygen. | Glucose is produce. CO2 is utilized in the dark reaction. |
| 7. | Photolysis occurs. | Photolysis does not occur. |
Difference Between Aerobic Respiration And Anaerobic Respiration
| S.No. |
Aerobic Respiration |
Anaerobic Respiration |
|---|
| 1. | It is that type of respiration in which oxygen is necessary. | It takes place in the absence of oxygen. |
| 2. | It oxidizes the food completely. | It oxidizes the food partially. |
| 3. | During this process large amount of energy is released i.e. 2827 KJ. | In this process, small amount of energy is released i.e. 210 KJ (in bacteria 8 fungi) and 150 KJ (in animals). |
| 4. | Carbon dioxide and water are the end products of this process. | The end products are lactic acid (in animals), Ethanol and carbon dioxide (in bacteria and fungi). |
By Mrs. Ayesha Arif
(Jauhar Progressive School)
LONG QUESTION & ANSWERS
Q. Write detailed answers of the following questions:
Source: Special Thanks To Sir Syed Arif Ali