Chapter No.4
p- Block Elements
Scientific Reasons
Q.1: Why Sulphuric Acid is a sulphonating agent?
Ans: Sulphuric Acid has a sulpnonyl group (-SO
3H). Wnen it reacts with an organic compound it replaces hydrogen with sulphonyl group Due to this property it is called Sulphonating Agent.
Q.2: Why Nitric Acid is an oxidizing agent?
Ans: Nitric Acid decomposes and gives atomic oxygen. which oxidizes substances.
2HNO3 ⟶ 2NO2 + H2O + [O]
That's why nitric acid is an oxidizing agent
Q.3: Why Graphite is a good conductor of electricity?
Ans: In graphite the outer most 3 electrons engaged in sp
2-hybridization, whereas 1 electron remains free causing conduction of electricity.
Q.4: Why atomic radii increase down the group in the p-block element? OR Why the atomic size of sulphur is bigger than that of oxygen?
Ans: Atomic radii size increases down the group due to addition of a new orbit it shell. That's why Sulphur is bigger than that of Oxygen.
Q.5: Why the metallic character or electropositivity of elements increase down the group?
Ans: The metallic character of elements is inversely proportional to the factors.
-
a) ionization potential
-
b) electron affinity
-
c) electron population
Hence metallic character or electropositivity increases down the group as these factors decrease.
Q.6: Why diamond is the hardest known substance?
Ans: Diamond is the hardest known substance as its valence 4 electrons are strongly bonded due to sp
3-hybridization. It has high C-C bond energy i,e. 347 KJ mol
-1. It has high refractive index as well as melting point too.
Q.7: Why hydrogen Sulphide is a gas where as water is a liquid?
Ans: The hydrogen bonding affects the physical properties greatly. The effect decreases down the group due to decrease of electronegativity. Hence H
2S is a gas having weak hydrogen bonding where as H
2O is liquid having stronger hydrogen bonding.
Q.8: Why Aluminium hydroxide acts both as a base and an acid?
Ans: The substance which can react with acids as well as bases are called "Amphoteric".
Aluminium hydroxide reacts with both acid and base as shown below:
Base (neutralizing an acid):
Al(OH)3 + 3HCI ⟶ AlCl2 + 3H2O
Acid (neutralizing a base):
Al(OH)3 + NaOH ⟶ Na[Al(OH)4]
Q.9: Why graphite is more stable allotropic form of carbon than diamond?
Ans: The radius of graphite is greater than Diamond. Atoms inside graphite have the autonomy to move around its lattice, therefore it has more entropy and more stable at higher temperatures.
Q.10: Why diamond is lustrous whereas carbon is dull-colored?
Ans: Diamond has high optical dispersion ability i.e. to disperse light into different colours which makes it luster. Whereas other allotropic forms of carbon do not nave such ability, hence they are dull coloured.
Q.11: Why electron population of aluminium is less than that of boron?
Ans: Electron population is defined as the ratio of outer most electrons to the capacity of outer most shell.
In Aluiminium:
13Al = 1s
2, 2s
2, 2p
6, 3s
2, 3p
1
No. of outer most electron = 3.
Capacity of outer most shell = 18 (M-shell)
i.e. electron population is 3:18
In Boron:
6B 1s
2, 2s
2, 2p
1
No. of outer most electron=3.
Capacity of outer most shell=8 (L-shell)
i.e. electron population is 3:8
Hence electron population of aluminium is less than that of boron.
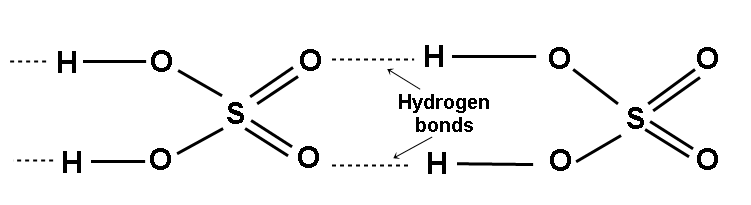
Q.12: Why the viscosity and boiling point of H2SO4 are high?
Ans: Sulphuric acid has 2 hydrogen atoms form stronger hydrogen bonding with each other causing greater viscosity and high boiling point.
Q.13: Why ammonia is a stronger base than phosphine?
Ans: The hydrides of V-A groups are basic in nature, but in Ammonia, N has greater electronegativity as compared to P in Phosphine. Moreover. ammonia is more soluble in water but phosphine is less. Due to reasons NH
3 is more stronger base as compared to the PH
3.